Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Topics covered: Enzyme catalysis
Instructor: Catherine Drennan, Elizabeth Vogel Taylor
Lecture 35: Enzyme Catalysis
Related Resources
Lecture Notes (PDF)
The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu.
PROFESSOR: Today is our final clicker competition. The first question is up. And I would just like to go over the current standing for the clicker competition as we're getting started here. So, recitation 6 has two wins, and there are other recitations and have one win, so recitation 2, 3, 5, 7, and 10 have one win. If any of those recitation wins today, we're going to go to a tie breaker to decide the overall recitation champion. There are also recitations 1, 4, 9, and 11, who are looking for their first win. This is their last chance today. So, if they win today, they can not be overall champions, but they will have snacks, and they will assure that recitations 2, 3, 5, 7, and 10 do not win the overall competition. So, you have a chance to foil those recitations. And if we need to go to a tie breaker, it will not be from current material, it's going to be past material or past things that were mentioned. So, just keep that in mind.
All right, and for the overall winner, I just would like to show you what you will win, so members of the recitation will each get their own chemistry t-shirt, and at some schools, of course, you would look at this and say oh, they're on the varsity team, but at MIT, it means that you are associated with course 5. So, Department of Chemistry at Massachusetts Institute of Technology on the back, and we have different colors and different sizes.
All right, so time to get started with the clicker competition. Go ahead and click in your first responses. OK, let's just take 10 more seconds. All right. Big improvement from the first time we asked this question last class where it's about 33%, now we're up to 89. And no, it is not temperature dependent. And we're going to be talking more about activation energy today.
All right, so today we're going to finish up kinetics, and we're going to start with the material that we didn't quite get to at the end of last class. And I love this part, because I love it when we can think about what we learned before in a slightly different way. So, when I first started lecturing, I was talking about LeChatelier principle and, of course, encouraged students to sort of -- you know it's not too intuitive to MIT students, but LeChatelier's principle says that if you apply stress to a system, it tends to respond in such a way to minimize that stress. So it's all about minimizing stress, which is something that's hard to kind to imagine this time of year, a little easier to imagine activation energy barriers, a little hard to imagine minimizing stress. But we're back to talking about minimizing stress. And we talked about adding reagents and removing products, and we talked about how reactions will shift to respond to stresses. And one of the things we talked about is rationalizing the shift of reactions at equilibrium when temperature has changed. So, if you increase the temperature of a particular reaction, it tends to shift in the endothermic direction to remove that added heat from the reaction.
So, that's what we learned before, but now we're going to think about this in a slightly different way. So we're going to think about this in terms of these reaction coordinate diagrams. So, on one axis we have p e plotted -- what does that stand for? Potential energy, right, so we have potential energy going up on both sides. And here we have a view of an endothermic reaction, and over here is an exothermic reaction.
So, let's just take a look at this. For the endothermic reaction, we have reactants and products, we have a delta e between those, and we have a very large activation energy barrier for the forward direction. And, of course, for anything to react, there always has to be some barrier, you need some amount of critical energy, because as the molecules come close to each other, and if they're going to react and form some kind of product, you have to sort of distort bonds and sort of rearrange things, and that takes a certain amount of energy. So if they come together and they have that energy, they'll react and go on to products, if they don't they'll break apart and go back and be reactants. And how much temperature the system has, is more likely that they'll be able to react. So it's more likely, if there's high temperature, they could overcome this activation energy barrier.
All right, so in an endothermic reaction, you have a big one in the forward direction, and a much smaller activation energy in the reverse direction. For exothermic reaction it's the reverse -- there's a smaller barrier for the forward direction, and a much larger barrier for the reverse direction.
So, we can think about the sign of delta e. So delta e, this difference here which is related to delta h for liquids and solids, it's pretty much the same for gases is 1% or 2% different. So you have a positive value here, and you can get a positive delta e here if you have a big number for the forward activation energy barrier minus a small number for the reverse, so that's going to give you a positive number. For an exothermic reaction, delta e's going to be negative, and you get a negative number if you take a small number here for the forward activation energy, and subtract it from a larger number here for the reverse activation energy. And remember, this is one of the equations that we're not going to give you, you need to have that memorized because it's part of understanding what's going on in these diagrams.
So now let's think about what increasing the temperature is going to do. So, it will help the molecules if the is temperatures increase, they'll have enough energy to overcome barriers. Now if the barriers are pretty small, then it's not very hard for the molecules to get over them, regardless of the temperature. But when the barrier is big, then temperature is going to make a big difference. And you can sort of think about this in your own life as you have a small assignment to do, you just jump in and do it and get it done and it's fine. But if it's a very, very large thing that you have to do, you often need to procrastinate longer before you start doing it. So, a small barrier's not so much of a problem, but if it's a big barrier, you need to increase that temperature to help the molecules get over the bigger barriers.
So, let's think about what this means in terms of the direction that a reaction might shift. So, if you increase a temperature over here with an endothermic reaction, it makes it easier to overcome the bigger barrier. The forward reaction barrier is the bigger one, so increasing the temperature will help molecules get over that barrier. They're already doing fine with the reverse barrier, it's the forward barrier that we're having trouble with. So, if you increase the temperature, that's going to shift the equilibrium toward products, or it will shift the reaction in the endothermic direction. That's what we had learned before with LeChatelier's principle, but now there's a new way of thinking about why that is true.
So, for an exothermic reaction, if you increase the temperature, that helps with the bigger barrier. Again, here the bigger barrier is for the reverse reaction. So, if you increase the temperature of an exothermic reaction, it's going to shift it toward reactants. More molecules can overcome that reverse barrier, and so you'll form more reactants, it'll shift toward reactants or it'll shift in the endothermic direction. Again, these are the same things that we already knew from LeChatelier, but it's a different way of thinking about why those things are true.
So, a large activation energy barrier means that rate constants are very sensitive to temperature. And so, if you have a big activation energy barrier, increasing the temperature makes a big difference -- if it's a big barrier, then there's going to have a big difference if you have higher temperature. If it's a small barrier, then increasing the temperature doesn't make much of a difference. And so you can think about that in terms of these diagrams as well.
So, now we're done with temperature and we're going to talk about catalysis and the use of catalysts. And so, if you remember there were factors affecting the reaction, and we've talked about everything on this list except for catalysts. So today we're going to talk about catalysts.
So, a catalyst is a substance that speeds up a reaction, but it doesn't get consumed in the reaction, it doesn't undergo any permanent change itself. It's just added to speed up the reaction. So catalysts do not appear in the overall balanced equation. So let's look at what a catalyst is going to do, and we're going to look at in terms of these potential energy diagrams as well. So here we here we have a reaction, we're going from reactants down here to products. We have the delta e for the reaction, we have a forward activation energy barrier and a reverse one.
So, up here, at the top, that's our barrier without a catalyst. This is the transition state or the activated complex up here, so you have to overcome the barrier, you form some kind of activated complex, which then, if there's enough energy to overcome that barrier, goes on to products. So when you add a catalyst what happens is it lowers this barrier. So, in the blue line here is the new activation energy barrier. This is the barrier with a catalyst, and so this would then be the new transition state with a catalyst.
So when it lowers the barrier, it's going to lower the barrier for the forward reaction, so we have a new activation energy for the forward reaction, and we're going to have a new activation energy for the reverse direction. So catalysts work by reducing both the forward and the reverse activation energy barrier. And another way that you can say this is that they stabilize the activated complex or the transition state. So here you have an activated complex with a much higher potential energy without the catalysts, and by stabilizing, you lower in energy that transition state. So that's another way of expressing what a catalyst does.
So, catalysts have no effect on the thermodynamics of the reaction, they affect the kinetics of the reaction, but they don't affect the thermodynamics of the reaction. And so, of course, when you think of thermodynamics, you often think of delta g. So, why don't you tell me what you think, what would happen about, so delta g is a state function, it's independent of path, and so therefore, what can you tell me about the equilibrium constant in the presence of a catalyst? OK, let's just take 10 more seconds. Yup, very good. So, the equilibrium constant is not changed. The thermodynamics, which includes delta g and the equilibrium constant are not affected, the rates of the reaction are affected.
All right, so opposite of a catalyst is an inhabitor, and we'll talk more about this at the end of class, so it would -- inhibitor is slow or sometimes stop the rate of the reaction, and one way that they do this is by increasing the activation energy. So let's consider types of catalysts now. You can have a homogeneous catalyst, which is in the same phase as the reaction that it's catalyzing as the reactants. An example of this is depletion of the ozone layer by chlorofluorocarbons. And so, this was one of the big environmental challenges that the U.S. has faced, whether it should ban these chlorofluorocarbons, and there was a lot of debate on what the data really was about what how much they affected the ozone layer. And I guess that debate is somewhat still going on, although I think most people now recognize that this is a serious problem and that legislation is really needed to help correct it. So that's an example. In this case they're all gases, so that's homogeneous catalyst, not a happy one.
You could also have heterogeneous catalysts, which are a different phase. And here's another example that has to do with the environment. So a catalytic converter is an example of a heterogeneous catalyst. So here you have a solid metal surface, you can have palladium or platinum that will catalyze reactions with gases, and so they catalyze oxidation of hydrocarbons, carbon monoxide, also reduction of nitrogen oxide. So this is all to reduce pollution. So that's an example of a heterogeneous catalyst. And let me just show you a little movie of how that might work.
So in this movie, in grey here we have a metal surface, and this metal surface can break the hydrogen bond of h 2. And so here, it is already broken, the h 2 bond, so there's a little hydrogen there and hydrogen there, and so that activates the hydrogen to react. And so, then we have ethene molecule come in, and oh, there goes the hydrogen, and oh, there goes the hydrogen, oh, there goes the other one and you can form ethane. So it speeds up the reaction by breaking the h 2 bond so it's more ready to react. That would be an example of a heterogeneous catalyst there.
All right, and, of course, you may all have guessed that my favorite kind of catalysts are enzymes. They are the catalyst of life. And so, enzymes are made up of protein, or mostly protein molecules -- you can have an enzyme that's actually made of RNA, but most are protein molecules. And they're typically about 20,000 grams per mole or more, and they're capable of carrying out specific reactions. And so, they're made up of amino acids, and just to take a quick look at that, an amino acid has an amino group and it has a carboxyl group, and it has what's called an alpha carbon that has a side chain on it, which is abbreviated r, and there are 20 different types of r. The simplest is just a hydrogen, you could also have a hydroxide, etc.
And so, this makes up the alphabet of proteins, there are 20 different ones of these that get connected via a peptide bond. So at the end of this amino acid you'd stick on the amino group of another amino acid and form this peptide bond. And then you put together hundreds and hundreds of these to form an enzyme complex. And so, the long chain of amino acids folds up and forms a compact structure.
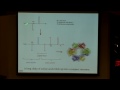
So, in this particular picture, there are about 200 amino acids in each of these colored units, so this is a tetramer, so there are four -- there's green, red, yellow and blue. And so, this is what's called a ribbon diagram. So, these ribbons, this is an alpha helix -- the beta strands are long. It traces out the position of the alpha carbons. And overall, this structure is about 90 angstroms by 70 angstroms by 50 angstroms. Of course, 1 angstroms is 1 times 10 the minus 10 meters. So these are fairly small. These protein molecules are, of course, in your body.
And this particular one is an enzyme that makes an antibiotic, and that antibiotic is fosfomycin, shown here. And so, fosfomycin is used in antibiotic combination therapies to treat staph infections and other kinds of very difficult infections to treat. And so, I always talk about the things that I'm most concerned about. There are a lot of things that are big threats that I don't worry too much about. Antibiotic resistance is something I actually worry a lot about. I guess I go to too many meetings were they talk about problems. But the rate at which different kinds of bacteria are becoming resistant to antibiotics seems to be increasing. So it used to take a lot longer before a resistance would appear than it does now. And there really haven't been, I think since about 1980s or so, really new antibiotics.
So, we're still using the same antibiotics that we have been, which causes more things to become immune or resistant to those antibiotics, and I think this is really dangerous. And there's not enough money in it for the pharmaceutical industry to really go after this. And it's not just a problem for biological warfare, although that is a possibility, but also in hospitals, you go in for some kind of surgery, you have to worry about the fact that even if the surgery goes great, you might get an infection that could really compromise your health in the hospital. And so, whether you have a heart problem, if you have cancer, you become immune compromised, there's a lot, a lot of cases where people end up not dying of cancer directly, they end up dying of the bacterial infection that can't be treated. So this is really a huge problem. So I always like to let you know all the possible problems that you can solve in your future. This is one that I think is particularly important, and I hope some of you will focus on this, because we really need different kinds of antibiotics, or we need to change the way we do medicine in this country so that resistance doesn't become as much of a problem as it has been.
All right, so that enzyme made an antibiotic, so we like it, and let's talk about how that works. How that particular series of amino acids folds up to do that reaction.
So now we have some new nomenclature, and sometimes when people get into the biochemistry world they get scared because there are all these words that they don't know what they mean. So most of them are not -- they are things that you can relate back to something you already know. So, we've been talking about reactants, and when you have a reactant with an enzyme, it's usually called a substrate. So this is just another name for a reactant molecule. And then substrates tend to bind in what we call the active site of the enzyme, which is the part of the enzyme that's going to do the chemistry. So those are two terms that you'll hear about in biochemistry.
All right, so we have an enzyme, we'll call it e, it binds a substrate, we'll call that s, and then it forms an enzyme substrate complex, which we'll call an e s complex, for enzyme substrate. And then that enzyme substrate complex will go to enzyme and product, which we'll call p. And if you look in the state-of-the-art biochemistry books, these are the abbreviations you'll see in there as well.
So, this pains me greatly to show this cartoon, because I spend my career determining 3-dimensional structures of proteins, and so to describe it as a little squiggly is painful for me. But nonetheless, there you go. Also, the substrate is almost about as big as the enzyme. That is also usually not true. But nonetheless, here's a little cartoon. Here's our substrate binding to our enzyme, it's forming an e s complex. And then the enzyme is going to move the ears of the substrate around to form product, and now product is released. So, this is actually pretty simple in terms of writing a mechanism.
And so, what we can do is write a mechanism the way that we have been writing a mechanism so far in this class. And so we're going to go through this, we're going to derive expression mostly to show you that all the things you've been learning are related to chemistry, they're also related to biochemistry. So, you already know a lot of biochemistry from just studying freshman chemistry in 511-1.
All right, so we have 2 steps in our mechanism. We have enzyme binding substrate to form an intermediate enzyme substrate complex, which then goes on to form enzyme plus product in step 2. So the first step is reversible, the second step is not as drawn. All right, so now we can come up with the rates for each of the individual steps in this overall mechanism, and since they are elementary reactions or steps in an overall mechanism, we can write the rate laws directly from what we observe.
So for the forward direction, we're going to have what rate constant? K 1 times the concentration of what? And? Yeah. So there we go. We have k 1 times the concentration of e and the concentration of s. See, you already knew how to do biochemistry. All right, so now at the rate of the reverse direction is -- what's our rate constant? K minus 1 times e s. And then for step 2, we have what rate constant? K 2 times e s.
All right, so now we can talk about the rate formation of product. And so you'll see this expressed as d concentration of p d t, so the change in product that's being formed and that's going to be equal to the second step here, k 2 times e s. But, as has been the case before, e s is an intermediate, and we need to solve our rate laws, our rate expressions, in terms of reactants or products and rate constants. So we need to solve for the intermediate.
All right, so let's think about solving for the intermediate. And why don't you tell me how to do that. OK, let's just take 10 more seconds. Very good. All right, so let's just take a look at why that is the case. So, when we solve for this, it's going to be equal to the formation of the intermediate, which happens in the first step with this rate that you told me, k 1 times the concentration of e s. And then we have the decomposition in the reverse of step one, so that's minus k minus 1 e s. And then we also have the consumption of the intermediate, which is -- so we have minus this step, which is k 2 times e s. So again, formation minus decomposition minus consumption.
And now, we can use the steady state approximation. So the steady state approximation applies in biochemistry just as well as in all of your chemistry problems that you've done in this course. And so, the steady state approximation allows us to set that whole term equal to zero. So it says that the net rate of intermediate formation and decomposition and consumption is 0, or you can express it as the rate of formation of the intermediate equals the rates of decomposition and consumption -- those are equivalent. So we can set all of this equal to zero. So again, the same as what we've done before.
All right, so now we're going to have a slight change of what we've done before, and this has to do with the practical consideration. So you can solve for these in terms of reactants and products and rate constants, but it's actually easier to solve for e s in terms of your total enzyme rather than your free enzyme. So what we've had before is our free enzyme, but we often don't know how much enzyme is free, and by that we mean not bound to the substrate. And so, we know how much total enzyme we put in, but we might not know how much of that is unbound. So it's actually easier for the experimentalist to solve for things in terms of total enzyme. And so what we're going to do then is we're going to replace our free enzyme term with the following, total enzyme minus bound, and total enzyme minus the bound enzyme is the free enzyme. So we're going to do this change that makes it easier for the experimentalist.
So here's the term we had before, but now we want to replace this term of e with e to 0 minus e s. So we're going to put that, here's the term e up here, so we're going to replace that with e 0 minus e s. And then we're going to multiply that out, so we get k 1, e 0, s minus k 1, e s, and then the concentration of substrate here, and the rest of it is the same. So we got rid of our term e.
So now we can solve for e s. So this is what we just had, and so we're going to rearrange so the e s terms are on one side, so we have an e s term here, here, and here. So all that is going to be on one side, and then this other term will be on the other side of the equation. So now we have moved all the e s terms over here, so we've got rid of the negative numbers, we've added them all to the other side, and then on this side we just have this k 1 term left.
Now we're going to pull out the e s terms. So, we'll solve for e s. We'll pull that out, that leaves us with k 1 times the concentration of s, k minus 1, and then k 2. And now we're going to take this and divide by that term in parentheses, and so now we've solved for e s in terms of total enzyme substrate and our rate constants.
All right, now we're going to do another thing that's a bit different. We're going to introduce a term in biochemistry, which is k m, also known as the Michaelis-Menten constant, and so k m is equal to the rate constant for the forward/reverse direction, k minus 1 plus k 2 over k 1. So we're going to now try to use this in our expression.
So we're going to put this term in there, and so to have this term of k m in there, we need to have this divided by k 1. So we can do that, it's OK, as long as we divide everything by k 1, so we're going to divide the top by k 1, and this by k 1, and that by k 1. So now, we divide all by k 1, and so we have this top with k 1, this term with k 1, and this with k 1, and that's our k m value. But now we can simplify this, we've got a lot of k 1's here, and so if we simplify this, we can cross out those k 1's over there. And we can also get rid of these k 1's over here. And that's going to leave us with a much simpler expression. So that's going to leave us with the total enzyme times substrate over substrate plus k m. And that looks a whole lot better than that other term that we had before.
OK, so we can now take this and substitute it back into the expression we had earlier. So here we had the rate of product formation, k 2 times this intermediate, we solved for the intermediate using total enzyme and using this k m term. And now we just put all of this times k 2. And if you put all of that times k 2, you have this expression, which is the Michaelis-Menten equation. So the only difference of what you did before was that you solved in terms of total enzyme, and also, we have this new k m term.
And so now we have an equation that's used in a lot of biochemistry research to think about enzyme kinetics. So let's think about enzyme kinetics for a minute. This is what a plot often looks like -- we are looking at the product formed by an enzyme versus the concentration of substrate. And it often goes up. It's pretty steep, and then it starts to level off. So let's think about what's happening here.
So if you have low substrate, so that's way down here, not much substrate is available, adding more substrate will increase this rate significantly, you'll form a lot more product, it increases the rate of product formation. Because a lot of the enzyme is free and can react with substrate. So it goes up, but then the rate starts to level off. So when you got to higher substrate concentrations, adding more substrate does not affect the rate much, the rate is leveling off. And we say that the enzyme is saturated. All the enzyme that you have in there already has substrate going on, it's already doing the chemistry. So if you're throwing more at it, the enzyme can't bind that substrate, it's busy with a different substrate molecule. So all the active sites in the enzyme are full, and so not much happens. So this kind of graph is what you often see in enzyme kinetics.
Now let's think about that in terms of our Michaelis-Menten equation. So let's think about conditions when there's a lot of substrate, when substrate is much greater than k m. So here we have substrate and k m on the bottom of our equation, and what's going to happen if this term is much, much bigger than this k m term? What happens to the k m term? It goes away. It's really small compared to this, it's kind of insignificant.
So, if that happens, then you can cancel substrate as well. And so, that means that under conditions where substrate is much greater than k m, the rate is a much simpler term, it's just equal to k 2 times your total enzyme. And so this has a name, this is called Vmax or the maximum velocity of the reaction. So, under these conditions, when the substrate is much greater than that k m value, this expression simplifies and you get just k 2, your rate constant, times your total enzyme, and that'll give you the maximum velocity of the reaction. And so, that's just written again here. Vmax equals k 2 times total enzyme, and that equation will be given to you on an equation sheet.
So what's happening? What's happening is that you're up here. So when substrate concentration is much greater than k m, then you're in these conditions, and you're at some maximum velocity. So the velocity will only go, the rate of the reaction will only go so fast, it will saturate, it'll level off up here, and so you can calculate that maximum rate of that reaction.
Now let's consider what happens when your substrate concentration equals your k m. So down here, if k m is equal to substrate, then we're going to have two substrates down here. And so, we can write this, if they're equal, we can write this as substrate plus substrate, and then we can do some canceling. So this will be equal to 2 substrates and then we can cancel that out, and so we get the rate of product formation under conditions where substrate equals k m of 1/2 k 2 times the total enzyme concentration. And this is referred to the half maximal rate. Remember, the maximal rate was k 2 times this e knot, and so half of that is half the maximal rate here.
So, the definition of k m is the concentration of substrate for which the rate is half maximal. So if your rate is half of the maximum rate, that substrate concentration that gives you half the maximum rate, is the value for k m. Substrate concentration equals k m at the half maximal rate. So let's just look at what that meant. So if this is Vmax up here, half of Vmax is here, half of that value, and the substrate concentration at half Vmax is equal to k m.
So you can write those in on your diagram. Vmax is this velocity up here, half of that, when you're at half of the maximal rate, k m equals the substrate concentration. And there will be problems that you will work in your book very shortly, and often, the problems just have to do, they'll give you the information in words and the calculations can often be pretty simple. Sometimes it'll say, figure out what the k m for this reaction is. At this substrate concentration, the rate is half maximal. Well, at that substrate concentration, that's the k m. So, a lot of the problems are sort of word problems, they give you the information and you need to know what the definitions are.
But let's try an example. So here in this example, we've talked about buffering in the blood, so the conversion of your ingredients that make your buffering agent in the blood, that can be catalyzed by an enzyme called carbonicanhydrase, and the following Michaelis-Menten constants are known, a k m is known, and a k 2 is known, and if you're doing experiment and you have a total concentration of your enzyme of 5 times 10 to the minus 6 molar, then you should be able to figure out what the maximum rate of the reaction would be. So, why don't you go ahead and tell me what the maximum rate would be. OK, let's just take 10 more seconds. Very good. Everyone's doing very good today.
All right, so all you have to do, you have remember Vmax equals your total enzyme concentration times your k 2. And pretty much no one, hardly anyone was fooled by k m value there. All right, very good.
So there are extra problems on enzyme kinetics posted on the extra problems posted on the Web. So there's a few there, so you can see the type of problems, again, they're like that, they're pretty simple. If you remember the definition of k m, and you'll be given the equation for Vmax, you should be able to handle the enzyme/kinetics problems pretty well. Often, also I'll ask questions like explain why at high substrate concentrations, the rate does not increase very much, or why the graph levels off, or I might ask you to draw the plot and tell me about low substrate concentrations, what's true there, and high substrate concentrations, what's true. So those are the types of questions you're going to get on enzyme kinetics, they're actually pretty simple.
All right, so let's just talk briefly about enzyme inhibition. So the opposite of catalysis -- instead of binding a substrate, you'll bind an inhibitor. So often, these are actually pretty simple. An inhibitor sometimes will look a lot like a substrate or like a transition complex, and it'll blind to the enzyme. And when you have that inhibitor stuck in the active site, substrate physically can't bind, so it occupies the place that substrate goes, substrate will come in but it can't bind anywhere. So this is actually the mechanism by which a number of pharmaceuticals work.
So, one thing that people who were designing enzyme inhibitors think about is the fact that enzymes do tend to stabilize a transition state in the reaction. So if you make an inhibitor that resembles a transition state, it should bind to the enzyme more tightly than either the reactants, the substrates, or the products. So, a lot of the pharmaceutical industry likes to try to figure out what the transition state might look like, and then try to make a molecule that looks like that transition state that hopefully will blind to the enzyme active site and prevent the enzyme from doing what it's supposed to do.
So, just to kind of look back at this figure for a minute that you had earlier in your notes, so you have the transition state is often stabilized by a catalyst, and so an enzyme will also stabilize a transition state. So if you make your drug look like a transition state, it will hopefully bind very tightly. And so this is one of the sort of principles that's behind a lot of the pharmaceuticals that have made to treat HIV infections.
So I mentioned last week we had world AIDS day, that understanding kinetics was actually very important in HIV research. And so, many of the pharmaceuticals that are given to HIV patients are what are called protease inhibitors.
So they inhibit enzymes that are called proteases, and if you have ase at the end of the name that means it's an enzyme, and so protease means that it's an enzyme that cleaves proteins. It's a protein ase. And so, how do these enzymes work. Well, they cleave other proteins, they cleave peptide bonds, and so you often have some kind of either activated water or other hydroxide molecule that will attack here the carbonyl of a peptide bond, and it forms a tetrahedral intermediate, which then collapses and it cleaves that peptide bond. So the peptide bond is then broken, so that's what a protease does, and a protease inhibitor prevents that cleavage.
So often, protease inhibitors look like tetrahedral intermediates, and so they'll bind to the protease active site and prevent the chemistry from occurring. So if it's a tetrahedral intermediate, what kind of angles should some pharmaceutical company be looking for in its compounds? 109 . 5, yes. So, molecules with this stable tetrahedral intermediate somewhere on the molecule could bind to the active site and prevent a catalysis.
So, let me just show you one example of an improved HIV drug, and there it is. This is the tetrahedral site that binds at the active site of that enzyme, and the enzyme can't cleave this tetrahedral intermediate, and so this just binds, but the enzyme can't work on it, so it just sits there and prevents substrate from binding. So that's how a lot of these compounds work. And so, this is just a little picture of the enzyme active site and there's an inhibitor bound to the enzyme active site, and so a number of companies are working on trying to come up with better and better inhibitors that will sit in the HIV protease active site. So, knowledgeable of a reaction mechanism can lead to new therapeutic treatments. So, one question with this, with protease, there are a lot of proteases, not just the ones involved with HIV, and so one real problem is specificity and toxicity, so you want to have a drug that inhibits one enzyme and not all the enzymes in that category. So that's a big problem in the pharmaceutical industry.
So, apparently, clicker results, we actually need to break a tie. But we can't do it now. OK, so Justin's section, woah! So, apparently we're having, we have the questions ready, but apparently they're not ready to be used right at the moment. So, we're going to use low tech here and indicate the results for today. And then on Wednesday, last day of class -- oh, my goodness, so close. Recitation 6, you were second.
All right, so on Wednesday then, we're going to have the tie breaker, and review and evaluations.
Free Downloads
Video
- iTunes U (MP4 - 105MB)
- Internet Archive (MP4 - 106MB)
Free Streaming
Subtitle
- English - US (SRT)