Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Topics covered: Entropy and disorder
Instructor: Catherine Drennan, Elizabeth Vogel Taylor

Lecture 17: Entropy and Dis...
Related Resources
Lecture Notes (PDF)
The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu.
PROFESSOR: So again, this is one more question on hybridization. This is the last question you'll get on hybridization before you see the exam. So now we're talking about hybridization of two different atoms in a molecule. So we're looking at this carbon atom here and this nitrogen. So using the rules that we've learned in terms of doing this quickly, let's see if you can get these answers in quick. So let's go ahead and just take 10 more seconds on this question here. Your parents are free to help if they're here with you.
OK. Excellent. excellent job, 98% of you. You're a lot louder with the parents here, so let's make sure we're still listening.
OK, so let's go over what we just we just established here. Carbon a is going to be s p 3 hybridized. What is the geometry, what's the vsper geometry there? Good, it's tetrahedral. And this nitrogen b here, that's s p 3 hybrid as well. What's the geometry around that nitrogen? Trigonal pyramidal, right. We have to take into account the fact that there's that lone pair there.
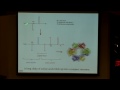
All right, so let's go ahead and switch over to the lecture notes and see what we just did here. And essentially actually what we just did was I had you identify two of four components that make up what's called the morphine rule. And the morphine rule is a set of structural elements that are responsible for the biological activity of morphine and other morphine-like drugs that have a similar pharmacological activity in terms of how they're active. So specifically, the morphine rule is a set of four components, which start with the phenyl ring here, so, an aromatic ring. The second thing you identified, which is an s p 3 hybrid carbon. And then following that we have a c h 2 c h 2 group here. And the last part of the rule is this s p 3 hybridized nitrogen.
So, in terms of thinking about what this means, I said this is responsible for the biological activity of morphine. If we take a look at the morphine molecule here, what you can see is that it's a little bit more complicated than this structure that we see right here. But if I go ahead and highlight the residues in purple, you can see that it, in fact, does follow the morphine rule.
And what this means is this is what allows morphine to actually bind into its receptor, which is a pain receptor and block the feeling of pain. So that's a very important thing in terms of thinking about hybridization in geometry, because we've actually established a very important structure and shape, which is this morphine rule here, that if it's found in a molecule, as long as there aren't other parts of the molecule that are messing up that interaction, we can actually form a very tight binder into these pain receptors.
So morphine, as you know, is a very potent pain killer. But you also, I'm sure, know that it's also very addictive, so it's only used sparingly in terms of treating pain in a general sense, and it's always used in very closely-monitored situations in terms of hospital care. But what's interesting to take note of is that the bioaction of morphine is very similar to endorphins that we naturally biosynthesize. Endorphins have a structure that's very similar to this structure up here -- endorphins are small peptide molecules that we biosynthesize and have in very low concentrations in our brain. They bind to pain receptors and block those pain receptors.
So, when someone talks about an endorphin high, which you sometimes get -- one example would be the runners' high, if you run for a long way running fast, eventually you'll hit that runner's high where you get this burst of endorphins. All of a sudden your feet don't hurt, your shins don't hurt, the pain goes away and you got this feeling of eurphoria. It's the exact same interaction and it's because of that structure there. It's not exactly the morphine rule in endorphins but it's very similar.
So, if we look at other derivatives of morphine, such as codeine and diacetylmorphine, these also you can see have this morphin rule within them, this structure that you've identified using your vsper rules and thinking about hybridization. Codeine, as you may know, is less of a potent pain killer compared to morphine, but it's also less addictive. So for that reason it's prescribed with slightly less oversight. So if you get your wisdom teeth removed or something like that, some of you might have had taken codeine before, and now you can think about by looking at the structure, a little bit more about how that worked in terms of blocking your pain.
One that I just want to mention because it's so interesting is this derivative, which is diacetylmorphine. The only difference between morphine and diacetylmorphine is the changing of an alcohol group, or two actually, two acetyl groups here. This was synthesized by the company Bayer, and you probably know Bayer from Bayer's aspirin. Bayer had a huge success with aspirin where they took salicylic acid and replaced an o h group with an acetyl group here, and made acetylsalicylic acid. Does anyone know the common name for that? Yup. So that's aspirin, and we often think of Bayer's aspirin. We associate it really closely. They worked really hard in marketing to make that really close association and with copywriting, so that when we think about aspirin we think about Bayers.
Bayers also made this change here, and they did find that diacetylmorphine is much, much more potent than morphine is. They could use just a tiny, tiny amount of this compound and get the same pain-killing properties that they saw with morphine. The problem is that this masked some of the side effects that they didn't realize initially, which was the extreme, extreme ability for you to become addicted to diacetylmorphine. And this was first thought of as a hero drug because it was such a strong pain killer, and this was called a Bayer's heroin. We don't usually associate the name Bayers with heroin, like we did with aspirin -- that's because Bayers did not work so hard to keep that association there. But it's an interesting story from the history of drugs.
One more example I want to show you is demerol, which is used clinically quite often. Demerol, if we look at the structure here, actually looks nothing like morphine at all. So to an untrained eye, you might not see the relationship there. But because you all know some of these basic principles of chemistry, you should be able to pick out that morphine rule right in the middle of demerol. And, in fact, demerol is a pain killer, it's an alternative to using morphine. Again, not quite as strong as morphine in terms of pain killing abilities, but also not as addictive either, and there are some other side effects, such as nausea that are limited with demerol compared to morphine. Demerol has its own set of problems, so it's not the perfect solution to a different alternative to morphine, but you do see it used in certain cases.
All right, so that's thinking about vsper theory, and that's thinking about hybridization. We're now going to shift gears to talking about some new topics today. So, we ended in Wednesday's lecture with giving that set of rules to very quickly determine hybridization. And then after we'd established that, we moved on to starting to talk about a little bit of thermochemistry. Specifically what we were talking about is the enthalpies of chemical reactions, so either the amount of heat that's released, or the amount of heat that's required in order to have a chemical reaction go. And we'd established so far two ways to think about how we can measure what the overall enthalpy of a reaction is.
And I just wanted to introduced one more technique that we can use to do that. And this relies on the fact that when we talk about enthalpy or we talk about the heat change of a reaction, this is a state function, and any time we're talking about state function, it's independent of path. So essentially, all that we're worried about is the state that we started in. So, for example, when we were talking about the oxidation of glucose, the state that we start in are the reactants, so it's glucose plus oxygen. And what we ended up with were six moles each of carbon dioxide and water. So, we don't care how we get there in terms of making this calculation, all we're interested in is the difference between those two states, to think about the overall change in enthalpy.
So, for example, when we talked about how we could calculate this, one way that we can do it, and we'll look at these numbers even more specifically in a second, is thinking about instead of going straight down here, which we might not have the numbers available to us to calculate directly, we can think about well, what happens if instead we break apart this glucose molecule and decompose it into its elements, because using tables in the back of our book, for example, we can figure out what the change in enthalpy is of this reaction here.
That doesn't quite get us to where we need to go though, so then we can think about the enthalpy of formation of six carbon dioxide molecules. And then we can go one step further and think about the enthalpy of formation for six water molecules. So this is just a round about way of going from here to here. Instead we went up and then down and then down, but because it's a state function, we're absolutely allowed to do this.
And to formalize this, we can talk about what's called Hess's law. And Hess's law just tell us that if we have two or three or any number of chemical reactions that we can add together in order to get the chemical equation that we're actually interested in, we can figure out the change in enthalpy of that reaction that we're interested in, just by adding together all of the other enthalpies of reaction for every other reaction that we had to add together in order to get there.
So, let's take a look at what we're talking about here. So when I just showed you that example graphically, I'm just writing it out more like an equation here, because the nice thing about Hess's law, is it allows you to think about chemical reactions kind of as if they're algebraic expressions. So we can just add all of these different expressions together, and if we're thinking about algebra, we can just think about crossing things out that are in the reactants versus in the products. So if we do this, what we end up getting is the reaction that we're interested in, the oxidation of glucose to form carbon dioxide in water.
So let's look pretty carefully at exactly the fact that these all cancel out to give us the reaction I say we get. So, for example, we can think about the oxygen molecules -- we can cancel out 6 here and 6 here, as long as we multiply this whole reaction by 6. Again, we can get rid of those last 3 oxygen molecules there. Similarly, we can cross out our 6 hydrogens from the products with 6 molecular hydrogens from the reactants. And then the last thing we can cancel out are our carbon atoms -- we have 6 in the products, and we have 6 in the reactants here. So you'll see that the only things that are not canceled out is what's left in our bottom reaction. So we can go ahead and just add up all of these different reaction enthalpies.
In the first case what we're going to have is an enthalpy of 1260, and that's in kilojoules per mole of glucose. And the reason that this is positive is because it's essentially the reverse reaction of the change in enthalpy of formation. So it's going to be a positive number there.
We add to that the enthalpy for this reaction here, which is negative 393 . 5, but we need to remember to multiply that by 6, because we're actually adding together six of those individual reactions. And lastly, we also add together six of this final reaction here, which has an enthalpy change of negative 285 . 8.
So if we go ahead and just add up all of our individual enthalpy changes, what we're going to end up with for our entire reaction here, is a change in enthalpy of negative 2816, and that's kilojoules per mole of glucose. Does anyone remember from Wednesday's class, does this match what we had seen experimentally?
STUDENT: [INAUDIBLE]
PROFESSOR: Yeah, so this is the exact number that we calculated when we used heats of formation, and it's also the exact number that's seen in terms of experimental.
All right. So there's actually three ways we now have to calculate heats of the change in enthalpy of an overall reaction. So, we have these in our notes from last time and from finishing the end of this unit right now, but I'm just going to summarize them for you on the board, because there are a couple things that can get confusing that I want to make sure no one is confusing, particularly in the upcoming exam.
So what was the first way that we learned to think about calculating enthalpies of reaction?
STUDENT: [INAUDIBLE]
PROFESSOR: Um-hmm. So, it's talking about bond enthalpies. And when we talk about bond enthalpy, the symbol that we see for a bond enthalpy is usually delta h. And this is where all the confusion starts, because everything's delta h, it just depends on the subscript here, right, what kind of delta h we're talking about. In the case of bond enthalpy often you'll see no subscript at all. But sometimes you do see a subscript, which would then just be delta h sub b here.
So if we're trying to do this calculation based on bond enthalpies, for the delta h for an overall reaction, what we want to do is take the sum of all of those bond enthalpies of our bonds that are broken, and what we want to do is subtract from that the sum of all our bond enthalpies of our bonds that are formed.
All right, so let's think a little bit more about what this means. So, if we're talking about bonds broken, are we talking about reactants or products?
STUDENT: [INAUDIBLE]
PROFESSOR: . Reactants. So, essentially we're summing up the bond enthalpy of reactants and subtracting it from the products. All right, great. So that's our first strategy there.
The second strategy that we learned is thinking about bond enthalpies of reaction by using the enthalpy change in terms of the enthalpy of formation. So this one is sometimes a little bit more intuitive, because we're talking about the enthalpy change it takes in order to form a given molecule. So, if we're trying to do a calculation using enthalpies of formation, what we find is that delta h of the reaction is equal to the sum of the delta h of formation of products or reactants?
STUDENT: Products.
PROFESSOR: Products, good. Of products minus the delta h of formation, and this is the sum again of reactants. All right, and our third strategy is what we just talked about, which is Hess's law, and in fact, both one and two are applications of Hess's law -- specific applications, but any application of Hess's law you can use any way of adding up different equations to get the final enthalpy of the reaction.
The reason I wanted to put these two strategies, however, on the board right next to each other, is so we can just confront a point of confusion that happens a lot with students, which is why in the first case do we have reactants minus product, and in the second case we have products minus reactants.
So, you need to keep these two straight when you're doing your calculations in terms of enthalpies, and the reason is because the definition of a bond enthalpy when we're talking about bonds, is the enthalpy that it takes in order to break the bond, the amount of heat that goes in to breaking a bond. So it's typically a positive number, because if you have a stable bond you have to put heat into it in order to break it. So you end up having a positive bond enthalpy here.
In contrast, when we're talking about enthalpies of formation, now we're talking about is the change of enthalpy in order to form a molecule. So, for example, if we have a nice stable molecule with strong bonds in it, what we're going to find is that the delta h of formation, would that be positive or negative? So it's negative. So you'd end up with a negative delta h of formation. So because you're talking about a term that's negative here where it would be positive in the first case, you end up flipping signs to have it work out. So, if this doesn't make sense as I'm saying it right now, just go back in your notes and look through and make sure that you aren't going to get mixed up when you're using bond enthalpies versus enthalpies of formation here.
All right, so that's pretty much all we're going to say on enthalpy. So I really just want to stress, this is going to be the end of exam 2 material here, I said I'd be clear about it in class today. This is it. It's also written in your notes when you go ahead to study. So remember, in terms of exam 2, which I'll remind you is on Wednesday, it's going to be all the way from the material on lecture 10 up to this point here. So only through enthalpy, and it's also going to be on problem-sets four and five, and I'll mention that this afternoon, as I said, I'll post a bunch of extra practice problems, which are more review of these same concepts, so you can get even more practice this weekend and early next week to prepare.
All right, so that's enthalpy, that's the end of exam 2 material. But there's actually another really important concept that we need to talk about -- we really don't want to stop with enthalpy, luckily it's exam 2 and not the end of the class, because a really important concept to think about is spontaneous change. So thinking about whether a reaction is spontaneous or non-spontaneous. And when we think about a spontaneous reaction, I think this is a term many of you are familiar with, spontaneous just means that the reactions going to proceed in the forward direction without any kind of outside intervention. So thinking of a spontaneous process, can talk about a chemical reaction, but you could just picture, for example, putting a round rock on the top of a hill -- it will spontaneously just roll right down that hill.
So a spontaneous process also has direction, right, because the rock won't spontaneously roll back up the hill without putting in work. So essentially we're talking about a spontaneous process when something's going to happen without actually having to do anything else to force it to happen.
So let's think about in terms of chemistry what we're talking about is a spontaneous reaction -- that's the specific type of spontaneous process that we're interested in. So let's think about a few different types of spontaneous reactions, and see if we can come up with some idea of what's going to cause them to be spontaneous.
So one spontaneous reaction is written here, so this is just the oxidation of iron, or the formation of rust. This turns out to also be an exothermic reaction, it has a negative delta h of 824 kilojoules per mole. Another spontaneous reaction is written here, the combination of an acid and a base, which neutralizes each other. So we have a hydronium ion and a hydroxide ion interacting to form water. This is spontaneous, and again, it's exothermic. So, its delta h is negative 55 . 9 kilojoules per mole.
Let's think about some really relevant reactions in our bodies. So one incredibly important reaction, of course, is ATP hydrolysis where we have adenosine here, a triphosphate, so we have three phosphate groups here. That's called ATP. It has a total charge of minus four. So if we hydrolize this, one of the phosphate bonds here and lose a phosphate, we end up with adenosine diphosphate or ADP and that has a charge of minus three. Yup?
STUDENT: On our notes, this equation [INAUDIBLE].
PROFESSOR: Oh, no. OK, thank you. Which equation are we talking about?
STUDENT: [INAUDIBLE]
PROFESSOR: I'm sorry, what page are you on? Page two. So the one with h 3 -- OK. All right, let's go back to that. OK, so if you can fix this reaction in your notes here. I'll also fix it on the website, so if you don't want to fix it now I'll just re-post the notes. So it should be four irons plus three oxygens is two f e 2 o 3 solid. Thanks for pointing that out. All right, does everyone have that down? I know you had to flip a page there.
All right, so I'll post it in the notes if you didn't get it there. Let's go back to ATP hydrolysis here. So we're going from ATP to ADP, and that is a spontaneous process, and it's an exothermic reaction as well. So we find that it's negative 24 kilojoules per mole in terms of the change in enthalpy there.
All right, so at this point I just showed you three reactions where we have something that's spontaneous and it's also exothermic. And I could show you a countless number of other reactions where it's the same thing. It's actually quite common if you have an exothermic reaction that it's also spontaneous at room temperature. So we might start to draw the conclusion that, in fact, it's enthalpy that's responsible for whether or not a reaction is spontaneous or non-spontaneous. So it's very easy to get that impression, but let me show you a few more reactions before we draw any conclusions. Let me show you some more spontaneous reactions.
So, for example, the conversion of solid h 2 o to liquid h 2 o at room temperature, I think we all know this is spontaneous. Ice melts at room temperature, but it turns out that the enthalpy change is positive, it's 7 kilojoules per mole. Similarly, if we look at this reaction here, which is ammonium nitrate. This is a very commonly used fertilizer, a very commonly used nitrogen source in agriculture. If we think about solvating this and forming the two ions, ammonium ion and nitrate ion. Again, this is a spontaneous reaction, but what we find here is that delta h again is positive, it's also endothermic. So thinking about all of this, would you say that enthalpy is the key to spontaneity?
STUDENT: No.
PROFESSOR: No, it's definitely not the key to spontaneity. It tends to correlate in many cases at room temperature, but it is not the key to spontaneity. And the key to spontaneity is instead something called Gibbs free energy or delta g. And if we think about what delta g is, we can relate it to enthalpy, and this will sort of show us why there's often this correlation, because delta g is equal delta h minus this term here, which is temperature, and that's temperature times a change in entropy. And we'll talk very in-depth in just a few minutes about what entropy actually is, but for now I just want you to think about the fact that in addition to thinking about enthalpy there's this other term here that comes into play.
So when we're talking about what the sign about delta g is or free energy is, some of you might already be familiar with this, when we're talking about a negative delta g, is this spontaneous or non-spontaneous. So, maybe not so familiar, which is totally fine. So in terms of thinking about a negative delta g, that's going to be a spontaneous process. Any time you see a negative free energy, which just like when we talk about entropy, it's a similar idea, it means that free energy is released as the reaction progresses. That's going to be a spontaneous process.
So that means that if delta g is greater than zero, then what we're going to see a non-spontaneous process. And finally in the case where delta g is equal to zero, at this point we're at equilibrium, which basically means that there's no net change in either the forward or the reverse reaction in terms of thinking about whether something's spontaneous or not spontaneous, it's just at equilibrium we see no net change. And something I want to point out is that this reaction is valid only when where at constant temperature and pressure, which we will be throughout the discussions in this class in terms of using this equation. And also, it turns out that when you do most chemical equations, you are, in fact, at a pretty constant temperature, and a constant pressure in terms of most things are done in the open atmosphere, so that's really not going to change.
All right, so let's just talk very briefly about why it is that free energy is what determines spontaneity, and not this enthalpy here, not what the change in enthalpy is. And it really comes in terms remembering it in terms of the definition. When we think about delta g, what that is is that it's energy that's released or used in the reaction, but if it is released, that it can directly be used to do work. It's what we call free energy -- it's free to do other things.
Whereas when we were talking about enthalpy here, well, this is the amount of heat that's released when we break and form the bonds in the reaction. But some of it actually gets stuck in the molecules, and this is just a way to think about it, this term here is one way we can think about it, it's just some energy that's getting stuck.
So, for example, molecules, which we don't really go into in this class, have vibrational movements, they have rotational movements, there's energy associated with that that can sort of get stuck, as we could say, some of the enthalpy that's released in terms of forming and breaking the bonds in a reaction. So that's our case where we could see that we have a negative delta h, but we still end up with a positive delta g.
And again, I haven't really gone into what entropy is here yet, and we will just in a few minutes.
But first, I want to take a look at one of the examples that we talked about, which is the conversion of ammonium nitrate when its solvated, breaking up into its ions. So what I had told you is that, in fact, this is a positive delta h, but still that the reaction is spontaneous. So let's just do this calculation and see if we can confirm that in terms of delta g. So the reaction again that we're going to use is delta g equals delta h minus t delta s. So if we're talking about room temperature here, so we're going to say that delta g in this case is going to be equal to 28 kilojoules per mole, minus -- we're at room temperature or 298 kelvin -- in this reaction, you always put temperature into kelvin. And then we need to multiply it by the entropy, which is 109 joules per kelvin mole.
I want to point out that entropy tends to be a much smaller value than enthalpy, so it's reported in joules instead of kilojoules. When you solve these problems, it's really important to convert it into kilojoules so that your units work out and you don't get a completely crazy answer. So we want to convert this to 0 . 109 kilojoules per kelvin mole. So we can just re-write this, so we have 28 kilojoules per mole, then this term here will be 32 . 5 kilojoules per mole. So what we end up with our delta g for this reaction is going to be negative 4 kilojoules per mole.
All right, so talking about this as a negative delta g, would you say this is a spontaneous or non-spontaneous reaction. Yeah, it's spontaneous. So what we're going to see is delta g is negative 4, so this reaction is spontaneous even though our enthalpy change was positive.
Let's take a look at one more reaction since we spent so much time discussing the oxidation of glucose. So again, what we know about this reaction, we've already calculated the delta h is negative 2816 kilojoules per mole. And you could look up and we'll figure out how to calculate it soon, the change in entropy, which is plus 233 joules per k mole. So before we actually do this calculation, let's go to a clicker question and I want you to think about what's happening here.
So if you're thinking about the oxidation of glucose and what the sign is of the change in enthalpy and the change in entropy, which of these statements is true. Is it going to be spontaneous at all temperatures, non-spontaneous at all temperatures, or will it depend on the temperature whether this reaction is spontaneous or not spontaneous? So, remember this is our relationship here. So let's go ahead and just take 10 seconds on this one.
OK, so we have a pretty mixed response here. So this could have made or broke the chances of your team on the competition today, but they'll be a few more left to redeem yourselves if you got it incorrect. So what we're going to find is that it's spontaneous at all temperatures, and we can actually just look at this equation here to figure out why that is.
If our reaction is spontaneous, that means that delta g is negative. So in terms of delta h being negative, well, that's going to always contribute to delta g being negative no matter what the temperature is. And if delta s is positive, since it's minus t delta s, and our temperature is always positive because we're on the kelvin scale, so it starts at zero there, then this component here is always going to be negative as well. So we're always going to have two negative numbers so that our combination, our delta g's always going to be negative, it's always going to be spontaneous.
All right, so let's make sure that's what we do see when we calculate this for the oxidation of glucose here. So if we do this and we plug in our numbers, we see that delta g is going to be equal to negative 2816 times the temperature, room temperature, times again, remember to put 0. 233, because we need to convert from joules to kilojoules for our entropy term. So what we find out is that this reaction has a delta g of negative 2885 kilojoules per mole. This is a spontaneous reaction.
All right, so let's get back to that entropy term. I introduced it without explaining at all what it really is. So let's take a few moments and think about entropy. Entropy is actually a very easy concept to think about, it's a measure of disorder. I think most of us have a very good concept of how things tend to go to disorder, so it's something we can conceptualize just in an infinite number of ways. And entropy is simply a measure of the disorder of a system. And when we're talking about chemical reactions, what we're talking about is a change in entropy. So whether, as a reaction goes forward, are we becoming more ordered or are we becoming more disordered? So just like we saw for enthalpy, entropy also is a state function, so it doesn't matter in terms of path how we got from point a to point b. All we need to worry about is the current state of our system, what the current entropy is and take the difference.
So, an example that I like to use in terms of conceptually thinking about what's going on in terms of disorder, and which is especially relevant in New England is thinking about stone wall. So you can think about a stone wall in terms of being very, very ordered, right. If you just built your stone wall, every stone is in place, it has a high degree of order. So in other words, the disorder is very low. So it has a low entropy, a low level of disorder. But if we're looking at a New England stone wall, which are typically found if you're hiking in the woods you see ones that are just ancient and are completely crumbling down, you'll find that the stone wall is no longer quite so ordered as it was. In fact, let's say there's a few stones that have fallen off, our disorder has increased, so we end up increasing the entropy.
And I do just want to point out with this analogy that it is a state function. So for thinking about the change in entropy, which is this distance right here, we can either calculate it directly from here to here. but it also doesn't matter, let's say the wall crumbled down completely and we ended up having a very high degree of disorder, if then someone put most of the stones back in place and we went back down to this degree of disorder here, it doesn't matter, it's a state function, all we care about is the actual difference between our starting point and our ending point.
And I just can't resist putting in a little quote, "something there is that doesn't love a wall." Does anybody know where this quote is from -- very famous poem, very famous poet. Good guesses, parents can help with this. Yes, excellent. I've never heard the correct answer this question before. Excellent. So this is Robert Frost in The Mending Wall. He talks about, it sounds like a lot of you did know this, this makes me full of joy. "Something there is that doesn't love a wall," there's many interpretations for this poem, but maybe it's about entropy. Entropy doesn't love a wall, we're going from order to disorder, that wall wants to come down.
All right. So let's formalize these thoughts on entropy and in terms of what we're talking about. Again, what I said on that's chart there is as we increase entropy, we're increasing disorder. So if you saw a positive change in entropy in a reaction, would you think about that as being more or less ordered?
STUDENT: [INAUDIBLE]
PROFESSOR: Yeah, so it's actually going to be less ordered, and, in fact, I should stop saying order, I should be just saying disorder because disorder's actually what we're measuring, and there's an increase in disorder here when we have a positive change in enthalpy. So if we have a negative change in enthalpy, we're going to see a decrease in disorder.
So in terms of considering different types of states that we can be in, whether we're in a gas state or a liquid state or a solid state for any given molecule or any given compound, we can think about how much order or disorder these states have. So which of these three states would you call the most disordered?
STUDENT: Gas.
PROFESSOR: Yeah, it's the gas state. So it turns out that the disorder gas is greater than liquid., which is greater than solid. This makes sense and the gas molecules are free to bounce around, go wherever they want. Once you're in a liquid, they have a little bit more limitation, they can't go anywhere. But the molecules can still slide all around and pass each other. Once you're in a solid state, this molecule is not going to flip places with the other molecules there. It's going to be kind of stuck in its place within the solid.
So, just understanding this explanation of entropy that we're going to have an increase in entropy if we have an increase in disorder, allows us to make predictions about reactions even without doing any calculations, and we always like when that happens, because then once we do calculations, we can check our calculations and make sure they make sense with what we would be predicting.
So before we talk about how to actually do a calculation, let's go to a clicker question here and make sure everyone is on the same page in terms of thinking about changes in entropy or changes or delta s for the reaction. So let's say we're talking about the decomposition of hydrogen peroxide into water and oxygen. I want you to tell me if you think it's going to have a positive delta s, a negative, or zero, or maybe this will change with temperature as well.
All right, so let's go ahead and take 10 seconds on this one. OK, excellent. Great job with the questions today. So we are actually going to see a positive delta s. this should be very clear because we're going from two moles of liquid, to now having two moles of liquid, plus a mole of gas, so we're going to be increasing the disorder of our system here.
All right, so let's think about how we actually can calculate, though, delta s. So far I've just been giving you the delta s's for the reactions, but, as I'm sure you can guess, you won't be given that information, for example, on upcoming problem-sets, you'll actually need to calculate the entropy. So what we can do is we can actually calculate the entropy of a reaction from absolute entropies of individual molecules that we're discussing.
So this is very similar to how we calculated the enthalpy of a reaction by taking the change of enthalpy of formation, except we don't even have to worry about a change in entropy because we can talk about entropy as an absolute value. There's an absolute zero in terms of entropy where there is no disorder at all. And this is described for any molecule as being its perfect crystal at absolute zero. So this is what we're going to say there's absolute complete order, there's no disorder at all in this system. So what we can take to calculate the entropy in a reaction, is just to take the sum of the entropy of all the products, and subtract from that the entropy from all of the reactants.
So let's try this for the example that you just did on the clicker question, and make sure that our calculation matches up with our prediction here. So again, we're just going to take the sum of the entropy of the products minus out of the reactants. So if we talk about this, we're starting with our product, so our first product is water. And what I want to point out is you need to make sure you multiply this by 2, because, in fact, we have two moles of water that are formed here.
STUDENT: [INAUDIBLE]
PROFESSOR: OK, I'm so sorry. Yeah, so yikes, this should be water. You know what, let's just write this on the board. So we're going to talk about delta s of the reaction. So that's to be sum of the entropy of the products. So we're going to be talking about 2 times entropy of water, and we'll add to that our other product, which is molecular oxygen here, and we don't need to put a number out here because it's just one mole. So that's of o 2, and we'll subtract from that, the entropy of hydrogen peroxide here. And do we need to put anything out here for this?
STUDENT: 2.
PROFESSOR: So, yeah, because there's two moles there. So water plus oxygen minus hydrogen peroxide. Let's hope I at least got the values right. Yeah, and actually I think this is right, I believe this is the entropy of water. So OK, so the actual numbers are right here for what is written on the board and hopefully in your notes. So what we find out is that the entropy of this reaction here, the change in entropy, the delta s, is 125 joules per k per mole.
All right, so we can also -- well, first, we already thought about why delta s is positive. Delta s is positive because we're going from a liquid to a liquid and a gas -- we're increasing our disorder. So let's think about whether this reaction is spontaneous or not, and to do that, we actually need to go ahead and completely calculate delta g. So to do this, we can take our values here, and delta g is equal to delta h minus t delta s. So if we plug all of this in, we have our delta h is negative 196 kilojoules per mole, minus our temperature, and I wanted you to write this again, because I want to make sure you get in the habit of converting our joules to kilojoules. It's 0 . 125 kilojoules per kelvin mole. So we end up with a delta g of negative 233 kilojoules per mole. Is that spontaneous or non-spontaneous?
STUDENT: [INAUDIBLE]
PROFESSOR: Great. So the reaction here is spontaneous. So actually, this is probably a reaction that you're familiar with because a lot of us do have hydrogen peroxide just in our medicine cabinet. And if you go and look at the expiration date on your hydrogen peroxide, you will see that it does, in fact, expire. And you might think oh no, what happens when it expires, when it goes bad? All that's happening is it's turning into water. So I wouldn't drink it after its expiration date, but probably there's more water than hydrogen peroxide at that point.
But it also actually brings out a really important point about thermodynamics, which is that thermodynamics, in fact, tells us if a reaction happening in the forward direction is spontaneous or non-spontaneous. So, for example, we see that yes, it is a spontaneous reaction. But you'll note that thermodynamics tells us absolutely nothing about the timeframe of that reaction. So some reactions that are spontaneous take place in a matter of seconds or microseconds or less. Other reactions, such as this, to have an appreciable amount of that reaction go forward, it actually takes years. You'll notice that it's not just one day before that hydrogen peroxide expires. It's takes a really long time to get a significant amount of this reaction to go. So just make sure, we will get to talking about kinetics, which does tell us about the timeframe of chemical reactions. But don't confuse thermochemistry or thermodynamics with kinetics. It doesn't matter how huge our delta g is in terms of being if it's negative two or if it's negative two million, that still doesn't tell us how fast the reaction's going to go.
All right. So let's think about one more example here in terms of thinking about entropy. So another example we talked about was the melting of ice at room temperature. I think all of us agree that this is spontaneous, but that the enthalpy change is positive, it's an endothermic reaction.
So we can think about calculating the entropy here, and I'm happy to see that I did, in fact, put the products h 2 o here as a liquid, subtracting the reactant, which is h 2 o as a solid. If we go ahead and plug in those values here, what we end up with is a delta s of this reaction of 28 . 59 joules. This makes sense because we're increasing the disorder of the system, we're seeing a positive delta s here. And I just answered that question for you. We're going why is delta s positive? We're going from a solid to liquid, we're increasing disorder.
So, we can calculate delta g as well, and if we do this we plug in these numbers here. What we end up with is that delta g equals negative 1 . 57 kilojoules per mole.
So it is spontaneous, delta g is just barely negative. And what actually is the case, which we'll talk about more on Monday and thinking more and more about how temperature actually affects these reactions, but what we know about melting of ice is that it is, in fact, temperature dependent, right. Ice doesn't melt at just any old temperature. So that's where temperature actually comes into play here.
All right. So we have a case -- spontaneous, even though delta h is positive.
All right, so let's think also about how we can go about calculating the free energy of formation. So we know that we can use the equation that we saw in terms of the free energy the reaction, but what if we're actually thinking about the free energy of the formation of a particular molecule? So that's actually completely analogous to thinking about the enthalpy of formation, or it's basically the standard Gibbs free energy of formation. So this is talking about forming a mole of a compound from its most stable elements, from its elements in their most stable states at pressure of one bar and at room temperature.
So we know that we can tabulate it must like we tabulated delta h formation, but we can also tabulate it from this reaction here, which it looks like I already told you this reaction, which I did but I told you a more general form, which was for the free energy of a reaction. This is the free energy of a reaction, which is the formation of a certain compound, and this is the free energy of formation equals the enthalpy of formation minus t delta s.
So let's think about why it is important to be thinking about these changes of free energy in terms of forming a molecule, and it's important because the delta g information is actually a measure of how stable or unstable a molecule is relative to its element. So, for example, if something is really stable relative to its elements, we can think about whether delta g of formation should be negative or positive. So let's have you do one last clicker question here, and tell me what do you think, if delta g of formation, of actually forming a molecule is going to be negative, would you say that the compound is going to be stable or unstable relative to the elements that that compound is made up of?
All right, let's take 10 seconds on this. OK, interesting. So this is going to be another tie breaker for us. So let's go back to the notes and think about this.
So it turns out that it's going to be stable, relative to its -- the compound is stable relative to its elements. And the reason for this is if you think of the reaction of the elements forming the compound, if the delta g of that reaction is negative, that means it's going to spontaneously form the compound. It's going to go -- release energy when it actually forms that compound. So that's another way of telling us that the compound is more stable than its elements. So conversely, if we have delta g of formation being greater than zero, so delta g of formation is just delta g of the reaction where the compound is formed, then what we find is that it's thermodynamically unstable relative to its elements, because instead of releasing energy in that reaction, you actually have to put energy into that reaction in order to make it happen.
So, delta g of formation can give us an indication of whether we have a stable or an unstable compound.
Free Downloads
Video
- iTunes U (MP4 - 108MB)
- Internet Archive (MP4 - 107MB)
Free Streaming
Subtitle
- English - US (SRT)