Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Topics covered: Transition metals and the treatment of lead poisoning
Instructor: Catherine Drennan, Elizabeth Vogel Taylor

Lecture 27: Transition Metals
Related Resources
Lecture Notes (PDF)
The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu.
PROFESSOR: OK, we have the clicker question up. We're going to have another clicker competition today. The defending champs, there's Darcy's recitation. So, OK, let's just take 10 more seconds on this. OK, not too bad, 74%.
So, in this problem, the trick was just to look at the equation and figure out what's going on -- which element is being reduced, and which one is being oxidized. So this is something that you will be seeing on the upcoming exam. So we're going to talk about that in a few minutes, but first I want to answer the question from last time.
So, first, let's just, I know you've all been wondering about how vitamin B12 is reduced in the body. So, let's take a look at this now. So, if everyone can quiet down, let's get started. So, vitamin B12 is reduced by a protein that's called flavodoxin. And flavodoxin is a protein that has a cofactor which is a flavin, and that's also a vitamin B, as it turns out, they're both vitamin B's. So, vitamin B12 has a redox potential or a standard reduction potential of minus 0 . 5 2 6 volts, and that is a very low number for a biological system. And flavodoxin has a potential of minus 0. 2 3 volts. So, which of these things is a better reducing agent? So, which thing, you want to think about reducing agent, which thing would prefer to be oxidized and reduce something else? So, with the low negative number, B12 is a better reducing agent than flavodoxin, and just based on these numbers, vitamin B12 should be reducing flavodoxin, not the other way around. So, how does this work then?
So, in your body right now, you have a version of a flavodoxin protein, and you of vitamin B12 attached to an enzyme, methionine synthase, and for that to be activated, it needs to be reduced. So how is this happening? So, let's consider whether the reduction of B12 by flavodoxin is spontaneous. It happens in your body so one might think that it is. But let's look at that.
So, we can look at it the same way you've looked at all the other systems, we're talking about batteries, electric chemical cells -- you can use the same equations if it's a biological system as you can for any other system. So we've seen this equation before. We've calculated changes in the standard reduction potential for a cell, and we talked about e nought for reduction minus the e nought for oxidation, and we can use that same equation. So we can put it in in the biological context. The vitamin B12 is the thing that's being reduced, and the flavodoxin is the thing that's being oxidized, that's the reaction that happens. So, we can plug those values in. So we have minus 0 . 5 2 6 volts, minus a negative 0 . 2 3 volts, and if you put those together, you get minus 0 . 2 9 6 volts. So is that going to be a spontaneous reaction? No, it's not going to be. Your value for the e is negative, which means what is true about delta g? Positive, so it won't be spontaneous.
So, let's figure out how not spontaneous it's going to be. How much trouble are we all in? So we can use again, the same equation that we used for batteries that we've used in this course -- just because it's a biological system, doesn't mean the equation doesn't hold. So our equation for delta g nought minus n, the number of moles of electrons times Faraday's constant times the change in the standard potential. So we can plug it in. I can tell you it's a one electron process -- flavodoxin puts one electron into vitamin B12, so we have minus 1 times Faraday's constant times the cell potential difference you just measured, which is minus 0 . 2 9 6, and if you multiply those out, then you get 28 . 6 kilojoules per mole positive. That is a very big value -- that's not spontaneous and it's not a small number for a biological system.
So, why don't we all have heart disease and megaloblastic anemia? Those are problems associated when this particular enzyme is not functioning. So, what happens in this system is what happens in a number of biological systems. How do you drive something forward that is not spontaneous? And what you can do is put energy into the system to drive that non-spontaneous reaction. And in this case, the energy that's put into the system is from a molecule called s adenosylmethionine. And the cleavage of s adenosylmethionine has a delta g nought of negative 37 . 6 kilojoules per mole. So it's more favorable than the reduction of B12 is unfavorable, and so this drives this system. So, s adenosylmethionine is your friend, it helps in the body for B12 to be reduced so that you can function and be healthy.
So, many biological systems work like this. So what have we been calling something, a cell, in which an unfavorable reaction is driven by applying some kind of energy or current, what do we call that? Two types of cells we mentioned. One that has a favorable, it has a spontaneous reaction. A cell that has a spontaneous reaction is called what? Galvanic. And the other kind is called? Yup. So, electrolitic cell. So this is sort of the biological equivalent of an electrolitic cell where you have s adenosylmethionine cleavage coupled to an unfavorable reaction to drive that reduction of B12 by flavodoxin. And B12 has such a low potential, that there really isn't anything else that can reduce it. It has one of the lowest potentials known in a biological system, so nature said, OK, well, we're not going to make something with a lower potential to do this chemistry, we'll have something else with a higher potential, but will drive the reaction because it's going to be non-spontaneous. So that's how this works.
So, today there's a long list of topics, all of these are pretty short and basically constitute the introductory material in this unit. And we've jumped ahead, this is in chapter 16.
So, I really like transition metals, because I am a fan of metals that are involved in biological systems. So, I just thought as part of chemistry, I just want to sort of review the kind of basic areas of chemistry for a minute. This is not in your handout, but just so you sort of think about what are all parts of chemistry. There is organic chemistry -- does anyone know what organic chemistry concerns itself with? Carbon. And then what we have known as inorganic chemistry. Anyone know what that is? Not carbon.
So, a lot of people who are inorganic chemists study transition metals, but it's basically other things besides carbon. And then one of the areas that I like is bioinorganic chemistry, and these are people who study metals in biology. So, people who study metals in biology, and we're also kind of referred to as, we're sort of in a club, which we call the MIB. So, some of you -- and Will Smith did a disservice -- people now associate this with hunting aliens, but in fact, people who are in MIB are associated with hunting for metal ions in biological cells. So this is sort of the true MIB. And in an honor of this discussion today, I am wearing a teeshirt from one of our meetings. This is from the International Congress on Bioinorganic Chemistry, which we refer to as ICBIC. And you notice that the clever people who made this teeshirt used the B from the bioinorganic to make B12, which is a very popular vitamin in the bioinorganic community.
So, metals in biology. Carbon, carbon's a good thing, amino acids are good, I like proteins. But often, when you take a metal and attach it to a protein, that protein can do really cool chemistry -- really need oxidation reduction chemistry. So, here is part of the periodic table that includes many of these transition metals that we're going to be talking about. And things that are in orange here are metals that are very important biologically. Some of the things that are in grey here are metals that are used as probes or as drugs in biological systems, and some could be both. So, when you add a metal to a protein you can do cool stuff. What can you do? Well, you can split nitrogen. You learned that the triple bond of nitrogen is pretty hard to break, but metals in proteins can do it. You've heard of hydrogen fuel cells and things like that. There's a protein called hydrogenase that uses metals to do chemistry with hydrogen. You could you radical based chemistry. You can do a lot of really great things when you have a protein that has a metal in it.
So, let me introduce you to some of the concepts and terminology involved in this, so we're going to have a metal and the metal's going to be bound to stuff. So, one of the great features of transition metals is their ability to form complexes with small molecules or ions. They also, this applies to a protein system in the case that instead of small molecules, you have amino acids of proteins it can also form complexes with.
So, the way that they do this is metals have often, they can attract electron density, usually a lone pair of electrons from another atom, and so they can form what are called these coordination complexes. So, now we're going to review for a minute, donor atoms, they're called ligands, and we can think about something that we learned when we talked about acids and bases, our more broad definition. So, donor atoms are ligands, and ligands are what? Lewis acids or Lewis bases, and what do they do? OK, let's just take 10 more seconds.
So most of you thought donor atoms -- the answers that had donate electrons did better, so that was good reading of the question, but they are Lewis bases. So Lewis bases donate electron pairs. So, here's some examples that you will see of ligands that we'll be talking about in this unit. You will become very familiar with some of these as you work problems. So they're typically donating one lone pair of electrons.
So, acceptor atoms are the transition metals themselves, and so the transition metals are acting as Lewis acids, and so then they would be accepting those lone pairs of electrons. So, you can think about coordination complexes as Lewis acids and Lewis bases, or acceptor atoms and donor ligands or donor atoms. So here are some examples of transition metals that we're going to see, so it's that sort of d-block of the periodic table that we'll become very familiar with in this unit.
So, then when you take your acceptor atom and your donor atom and you put them together, you get what is known as coordination complexes. So, coordination complex is just the metal or the Lewis acid surrounded by the ligands, or the Lewis bases or the donor atoms.
And here is an example of a coordination complex. We have metal, we have cobalt in the middle, and it's surrounded by a series of ligands, and these are n h 3 ligands. And so then our cobalt in the center, our metal, is the Lewis acid, and it's going to be the acceptor atom. And the n h 3 groups are our Lewis bases, they're donor atoms, see it's written with those two electrons there, that they're sharing their electrons with the cobalt forming this coordination complex. And in this scheme, we have the bonds are just these straight lines are in the plane, the thick bonds coming out are coming out toward you, and the dashed lines going back are going back into the screen here.
So, let's talk about a couple of definitions here. We have something called coordination number or CN number, and this is simply the number of ligands bonded to the metal. So the number is six, there is six ligands. Typically, the numbers will range for these coordination complexes to two to 12, with six being the most common. So, here is some notation. If you see this picture, you should be able to write a notation for it. And within a bracket, you would write cobalt, and then you have your n h 3, six of them, so you put that in a bracket and indicate that there's six n h 3 groups. Then you have this overall bracket here with a charge up above. And so over here, we have this little bracket plus 3, that means that this whole coordination complex has a charge of plus 3. Because it has a positive charge, sometimes coordination complexes are associated with counter ions. So, you might see three chloride minus 1 ions around to counter that charge. And if you did, you would see the three chlorides on the outside of the bracket. So things inside of the bracket are actually coordinated to the metal, things outside the bracket are counter ions. And so that would be how you would translate that particular notation.
All right, so, we're back to geometry again. As I said, everything you learn in this course, we're going to use again. So, if you're forgetting some material from units 1 and 2, this would be a good time to review those. So, if we have this coordination number of six things around, what kind of geometry are we going to have? All right, 10 seconds.
Yup, so we have octahedral geometry. And here is an example of our octahedral geometry. So, let's just quickly run through the rest of these. You can yell out the answers, either looking at your handout or not looking at your handout. The next one over here, what is it? Right, trigonal bipyramidal. For a c n number of five, we have another option shown over here, what's that one called? Square pyramidal. For c n numbers of four, there's two things that you'll commonly see, what's the first one called? Square planar. The second one? Tetrahedral. For c n number of three, what's that geometry called? Trigonal planar. And c n of two, only one option. Linear. All right, so let's just review the angles as well, those are not in your handouts, but you can yell those out as well. So what angle do we have in an octahedral system? 90. There are two angles involved if we have trigonal bipyramidal. What's the angle from the axial to the equatorial?
STUDENT: 90.
PROFESSOR: And what's the angle from one equatorial atom to another equatorial atom? So, we have 90 and 120. What about in our square pyramidal system, what are the angles here? 90. What about square planar? 90. Tetrahedral? Let's try that again with more enthusiasm. Awesome, 109 . 5. Trigonal planar? 120. And finally, linear. 180.
So, we're going to use this information again in this unit, and of course, it'll be on the final when we're talking about Lewis structures and hybridization and other things as well. So this comes back in several times, vsper theory, you have this several times.
OK. So, another term that you hear a lot when people are talking about coordination complexes is what's called the chelate effect. So, ligands that bind to a metal at one point are called unidentate or monodentate, and that's from dent, which is tooth, so one tooth. Now, if a ligand attaches with two or more points of attachment, it can be called a chelating ligand. And this comes from the Greek, chele is claw. So, if it's attaching with more than one point of attachment, if the ligand is grabbing on, it's like a claw and that's called a chelate. So, if you have two points of attachment, that's called bidentate. And even if you've never had a unit on transition metals before, I bet that you can answer this without me even telling you. What do you think tridentate might be? Three. Tetradentate? Four. Hexadentate? Six. So sometimes this would be a little question on the final. Please don't get this wrong, this is my gift to you, right, you knew it even before I taught it. So, this should be something that you can definitely get a couple extra points on on the final.
All right, so, chelates bind with more than one point of attachment, and metal chelates are unusually stable. And this property is due to favorable and tropic factor, so we're back to entropy, we're back to thermodynamics. And this has to do with the fact that when a chelate binds a metal, it releases a lot of water, and that makes the chelate pretty stable.
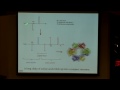
So, let me give you some examples of chelates, and then we're going to come back and think about the chelate effect again. Of course, you knew it, vitamin B12 has a chelating ligand associated with it. So, cobalt is in the middle of vitamin B12, and it has a ring system around it. And the ring system has these nitrogens, the nitrogens are the donor ligands to the cobalt, and so this ring system is attaching at four points, so it's a tetradentate ligand. There's also two other ligands attached to vitamin B12, there's an upper ligand shown here, which is 5 prime deoxyadenosine, and a bottom ligand, which is a dimethylbenzamitisol ligand.
So, let's take a look, this is the cartoon, but let's look at another model for this here. So, we have a ring system, we have this upper ligand, and then a lower ligand down here. And so, the middle ring system is a tetradentate chelate. Overall, at the cobalt metal, what is the geometry of this system? It's octahedral, right. So we have this sort of square, the middle part is square planar, but the two upper and lower ligands make the whole thing an octahedral system. So this is an example of a naturally occurring chelated complex.
So, this structure is actually fairly complicated for a vitamin. It's one of the most complex vitamins that's known, and I thought I'd just mention just a moment of history. So, this structure was determined by Dorothy Hodgkin who was a crystalographer in England. And she started working on this in the late 1940's, and people told her she was crazy, that something this large could never be solved by these x-ray defraction techniques. And, of course, now people are solving today's structures of things like the ribosomes. So, we've come a long way with crystallography. But she was one of the first true believers that x-ray crystallography was a powerful technique. And so, she went ahead, despite what everyone said, and determined this structure. And for this work and for some other things, she was awarded a Nobel Prize. So, this was a really significant contribution in the early 1950's and late 1940's. She had graduate students that she inspired. Some of them went on to become famous crystalographers. Other ones were not so successful -- crystallography's hard and it's not for everyone.
She was actually known also, Dorothy was, for her work in the third world and her liberal politics, but one of her graduate students did not agree with her politics, but they were still friends. So this is just a message that as you go through your career, Margaret Thatcher who is a graduate student of Dorothy Hodgkins, couldn't quite cut it as a crystalographer, but for some people, you haven't found your true thing, and she was able to find another job, so I'm told. So, just remember that if you're not good at one thing, there's something else out there that you might be good at.
So, let me give you a second example of a chelate. This molecule is known as EDTA. So this ligand has six points that it can attach to a metal. So, it is six atoms the can serve as these donor ligands. So, we have one up here, have the little electrons and the oxygen, another oxygen down here, nitrogen, nitrogen, another oxygen, another oxygen. So all of those six points can coordinate to a metal.
So here again is the free EDTA, and here's a structure of EDTA bound to a metal, the metal is abbreviated m. So, why don't you tell me what the geometry is of that metal? All right, let's take only 10 more seconds. Hoping for 90, we're getting close, maybe by the end of today.
All right, yeah, so it's octahedral geometry. So we have upper ligands and a lower ligand, two ligands coming out, two ligands going back, and it's also a hexadentate complex. So we see we're coordinated with the oxygen in red, nitrogen in blue, around to the other nitrogen in blue, another nitrogen in purple, another nitrogen up here in green and in blue.
So, EDTA, when EDTA binds to a metal, it forms a very stable metal complex. And the reason for this is that chelate effect, and it's entropy. So if we look, metals are often coordinated by waters if they're just is solution. But if you bind one molecule of EDTA to the metal, it'll take all of those six sites, displacing all of these six waters. So here, on this side, you have one thing free in solution, and over here you have six things that are free. So that's entrotropically favorable, you have more entropy when you have lots of more free things floating around. So this has greater entropy.
So, the chelate effect, then, has to do with entropic effect, that when you're binding a metal with multiple points of attachments, it's releasing water. So these are very stable. And so, because of this stability, they have a lot of important uses. What is one use you can think of that you might have for such a thing? When might you want to chelate a metal out -- get metal out of something pretty quickly. Yeah. Yeah, you can purify out a metal out of water with this. What about rushing to the emergency room with something? Yeah, lead poisoning, yup. So, sometimes you might want to just purify your water, you might be happy with that, other times you might really want to have that metal out. So, every emergency room in the United States, and probably mostly around the world, will have EDTA on hand in case someone comes in with lead poisoning.
Do you know who's at the most risk for lead poisoning, at least in this country? Kids. And what do they do they gives them lead poisoning? Lead paint, yeah. So, I don't know, most of you are probably living on campus, but if any of you move off campus, and some people who are living maybe across the river in fraternity houses, those buildings are old and they have had lead paint in at some point or another. So this is a big problem, actually, in the Boston area. So, if you have any toddlers visiting you, you might want to make sure they're not eating paint chips off any window sill or anything like that. Or if you do, have some EDTA on hand.
All right, one other thing, some of you, once you start studying chemistry, it's always fun/scary to start reading the ingredients on food packages. But you will discover that some food that you eat will say as an ingredient EDTA added for freshness. So, bacteria and fungi and things like that need metals for growth, metals are very important for life, and so you add a little EDTA and it prevents things from growing on your food. So that's added for freshness. So you'll see it in food.
Another use that occasionally I find MIT students are not as familiar with is in cleaning bathtubs, so you want to chelate out the calcium in tub scum, and that's a good use for EDTA.
So, I always like to mention how freshman chemistry information can make you a lot of money. So, I thought I'd tell you a little story about a man named Robert Black. So one day, I think it might have been a little before Thanksgiving, Mrs. Robert Black, I don't know her first name, said to her husband, "We're having company, how about you go clean the bathtub?" Apparently, Robert Black's wife had never mentioned this to him before, and he had, in fact, never cleaned a bathtub. Also, I think that Robert Black's wife was tired of cleaning the bathtub, and perhaps, had not done it for, say, a very long time. So, Robert Black went in there and tried to scrub off the scum, and it was actually really challenging and he was very frustrating, and he said, "I never want to do that again."
So, he went and developed a product, a shower cleaner, that had all the ingredients of the shower cleaners he was using, except that he advertised it slightly differently, and he said "Every time you shower, just spray a little bit on and you'll never have to really scrub again." because this will take care of it and avoid scrubbing in the future, so a little bit of spraying after each shower avoids these problems. And the ingredients he had in were all typical ingredients, including chelating agents. You need a surfactant to break up the water beads, and alcohol to get rid of the oily stuff, and the chelating agent for the calcium in the tub scum. And the one he actually used is EDTA.
So, as a result of a wife saying to her husband, it's your turn to clean the tub, they now make $70 million dollars annually. So, I think this is a lesson that we can learn in many ways that cleaning the tub is something that everyone should do at some point in their life, and that the simple things you learn in freshman chemistry can, if applied correctly, make you a lot of money. And I just want to encourage you, again, that anything that I teach you that you use to make a lot of money, I'll remind you that I am perfectly willing to have a cut of any of that. So, just something to keep in mind.
So, one more thing that you might have heard for EDTA. Have any of you heard, perhaps, maybe watching television or a movie, about a use for EDTA? Have any of you, perhaps, seen a movie called Blade? How many of you have seen this? Not very many people. Hmm, I think we're going to have a separate course in vampire movies and TV. Anyway, Blade fights vampires, and they have a special gun, and the special gun has cartridges -- cartridges are shown here. And the cartridges have in them -- anyone want to guess? EDTA, yes. And the idea behind it, if you shoot at a vampire, the vampire turns instantaneously to dust, and the idea behind it is that vampires drink blood, blood has what in it? Iron. What chelates iron? EDTA. And, therefore, if you chelate the iron out of blood, and a vampire's mostly blood, it just instantaneously turns to dust. So, actually, as chemistry goes, it's kind of cool, yeah. So, that's the final use that I am aware of for EDTA. Some of you may encounter some more.
All right, so coordination complexes. Some of them can have isomers. They can have geometric isomers. And geometric isomers can have vastly different properties. And if you're interested in biochemistry, this is something that you'll be interested in, because this can be very important in biological systems.
So, let me tell you about a coordination complex that has platinum at the center, it has two ammonia ligands and two chloride ligands, and it has two ways that you can do it. So, you can either put the ammonia ligands on different sides or you can put them on the same side. So, here we have two nitrogen ligands over here, and I have these here. So, on one side, you have the two nitrogen ligands, on the other side two chlorides, or we can have a transarrangement where they're across from each other. So you see, there's 2 different ways to do it, on the same side or sort of across. So, cis or trans. Cisplatin is a potent anti-cancer drug, transplatin has no use that anyone's been able to detect. So it's the exact same composition, but a different arrangement of the atoms coordinated to the central metal.
So, why should it make a difference, they have the same ingredients, why should one be a potent anti-cancer drug and the other one be useless? Well, it's because of how it interacts in the body. And so, one of the people who have spent a lot of their career studying cisplatin is Professor Steve Lippard, who's in the Chemistry Department here, and he has determined x-ray structures looking at this, and done a number of other studies. And so, here is a little cartoon of cisplatin bound to DNA, so the chlorides are displaced when it binds, and so they need to be on the same side, otherwise it's not going to work. And when cisplatin binds to DNA, then this will act to kill the cancer cells.
So, the chlorides both need to be on the same side so a combined DNA. If they're on other sides, that's not going to interact with DNA, and so it doesn't have any known function. So, geometric isomers, same composition, but in different arrangements can have vastly different properties.
So you can have what are called optical isomers or enantiomers, and so these can our mirror images of each other, but they're not superimposable. And so, when you have a mirror image like this, it's called a chiral molecule, and this is a term that you'll hear a lot when you take organic chemistry.
So, I have two mirror images up here, so these are mirror images, so the mirror plane is between these molecules. But these molecules may look the same in the mirror, but they're non-superimposable. So, they are, in fact, different molecules, and they can have different properties in a chiral environment. What do I mean by a chiral environment? Well, the human body, for example, is a chiral environment. So that's why a lot of drugs, people are wanting to make just one enantimer of the drug and not the other, one enantiomer can have good properties, the other one may not.
All right, so let's do some d-electron counting. This is the final thing we need to talk about in coordination complexes. So we're going to refer to a thing called the d-electron count of the metal, which just has to do with a group number from the periodic table minus the oxidation number of the metal, is going to tell us the d-electron count. So, if you haven't learned how to do oxidation numbers yet, you need to know that for exam 3, and you need to know that for this unit. So, let's look at a few examples of this, and we'll put up our friend, the periodic table. So let's look at the complex that we saw on the first page where we had -- in the beginning of today's lecture where we had cobalts with six n h 3 ligands in a coordination complex that had a charge of plus 3. So here, we need to figure out the oxidation number of the cobalt, and for this particular group, the overall charge of that is 0. We have three hydrogens at plus 1, and we also have a nitrogen at minus 1. And so, overall that charge is 0. So we have six things of 0, and what does that leave for our cobalt charge? Plus 3. Because overall, it now has to equal plus 3.
So, then our d count for our electrons is going to be the group number. And for cobalt, what's the group number? 9. So minus 3 is 6, so this cobalt in this complex is what's called a d 6 system.
All right, let's look at a couple other examples here. Let's look at nickel with carbon monoxide ligands, four of them. What would nickel be in this case? Someone said zero. That's right. So this is zero, and nickel is zero, the overall charge is zero. So, our oxidation number here is 0. So, our d count here, what's the group number for nickel? 10. 10 minus 0 is 10. So we have a d 10 system.
So, I'm going to write another example down and you're going to tell me the answer to this one as a clicker question. So we have cobalt, two waters -- three waters -- that's actually a mistake. It should be two. Two waters, because it's going to be six things -- this is not some kind of bizzaro seven complex. So here's the correct, cobalt, two waters, one ammonia, and three chlorides with an overall charge of minus 1. All right, let's just take 10 seconds, since we're running out of time.
So that's right, we should have a charge of plus 2 here. This is zero, 0 minus 3, overall minus 1, so we have a plus 2 state. We have 9 minus 2, which is 7, and it's a d 7 system.
Free Downloads
Video
- iTunes U (MP4 - 99MB)
- Internet Archive (MP4 - 99MB)
Free Streaming
Subtitle
- English - US (SRT)