Flash and JavaScript are required for this feature.
Download the video from iTunes U or the Internet Archive.
Topics covered: Wave-particle duality of light
Instructor: Catherine Drennan, Elizabeth Vogel Taylor

Lecture 3: Wave-Particle Du...
Related Resources
Lecture Notes (PDF)
The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu.
PROFESSOR: And this is a question based on where we left off on Wednesday -- we were talking about Coulomb's force law to describe the interaction between two particles, and good job, most of you got this correct.
So, what we're looking at here is the force when we have two charged particles, one positive, one negative -- here, the nucleus and an electron. So, I know this is a simple example and I can see everyone pretty much got it right, and probably those that didn't actually made some sort of clicker error is my guess.
But I wanted to use this to point out that in this class in general, any time you see an equation to explain a certain phenomenon, such as here looking at force, it's a good idea to check yourself by first plugging it into the actual equation, so you can plug in infinity and this equation here, and what you would see is, of course, the force, if you just solve the math problem goes to zero.
But you can also look at it qualitatively, so, if you think about the force between the electron and the proton, you could just qualitatively think about what's happening. If they're close together there's a certain force -- they're attracted because they have opposite charges, but as that gets further and further away, that force is going to get smaller and smaller, and eventually the force is going to approach zero.
So, it's a good kind of mental check as we go through this course to remember every time there's an equation, usually there's a very good reason for that equation, and you can go ahead and just use your qualitative knowledge, you don't have to just always stick with the math to check and justify your answers. So, we can get started with today's lecture notes.
And, as I mentioned, we left off and as we started back here to describe the atom and how the atom holds together the nucleus and the electron using classical mechanics. And today we'll finish that discussion, and, of course, point out actually the failure of classical mechanics to appropriately describe what's going on in an atom.
So, then we'll get to turn to a new kind of mechanics or quantum mechanics, which will in fact be able to describe what's happening on this very, very small size scale -- so on the atomic size scale on the order of nanometers or angstroms, very small particles. And the reason that quantum mechanics is going to work where classical mechanics fails is that classical mechanics did not take into account the fact that matter has both wave-like and particle-like properties, and light has both wave-like and particle-like properties.
So, we'll take a little bit of a step back after we introduce quantum mechanics, and talk about light as a wave, and the characteristic of waves, and then light as a particle. And one example of this is in the photoelectric effect.
So, we just talked about the force law to describe the interaction between a proton and an electron. You told me that when the distance went to infinity, the force went to zero. What happens instead when the distance goes to zero? What happens to the force?
Yeah. So, the force actually goes to infinity, and specifically it goes to negative infinity. Infinity is the force when we're thinking about it and our brains, negative infinity is when we actually plug it into the equation here, and the reason is the convention that the negative sign is just telling us the direction that the force is coming together instead of pushing apart. So, we can use Coulomb's force law to think about the force between these two particles -- and it does that, it tells us the force is a function of that distance. But what it does not tell us, which if we're trying to describe an atom we really want to know, is what happens to the distance as time passes? So, r is a function of time.
But luckily for us, there's a classical equation of motion that will, in fact, describe how the electron and nucleus change position or change their radius as a function of time. So, that's -- does anyone know which classical law of motion that would be?
Yup, so it's going to be Newton's second law, force equals mass times acceleration -- those of you that are quick page-turners, have a little one-up on answering that. And that tells us force as a function of acceleration, we want to know it though as a function of radius, so we can just take the first derivative and get ourselves to velocity. So, force is equal to mass times dv /dt.
But, of course, we want to go all the way to distance, so we take the second derivative and we have this equation for force here. And what we can do in order to bring the two equations together, is to plug in the Coulomb force law right here. So, now we have our Coulomb force law all plugged in here, and we have this differential equation that we could solve, if we wanted to figure out what the force was at different times t, or at different positions of r.
So, all you will have the opportunity to solve differential equations in your math courses here. We won't do it in this chemistry course. In later chemistry courses, you'll also get to solve differential equations. But instead in this chemistry course, I will just tell you the solutions to differential equations. And what we can do is we can start with some initial value of r, and here I write r being ten angstroms. That's a good approximation when we're talking about atoms, because that's about the size of and atom. So, let's say we start off at the distance being ten angstroms. We can plug that into this differential equation that we'll have and solve it, and what we find out is that r actually goes to zero at a time that's equal to 10 to the negative 10 seconds.
So, let's think qualitatively for a second about what that means or what the real meaning of that is. What that is telling us is that according to Newtonian mechanics and Coulomb's force law, is that the electron should actually plummet into the nucleus in 0.1 nanoseconds.
So, we have a little bit of a problem here. And the problem that we have is that what we're figuring out mathematically is not exactly matching up with what we're observing experimentally. And, in fact, it's often kind of difficult to experimentally test your mathematical predictions -- a lot of people spend many, many years testing one single mathematical prediction. But, I think all of us right now can probably test this prediction right here, and we're observing that, in fact, all of us and all the atoms we can see are not immediately collapsing in less than a nanosecond.
So, just, if you can take what I'm saying for a moment right now that in fact this should collapse in this very small time frame, we have to see that there's a problem with one of these two things, either the Coulomb force law or Newtonian mechanics. So, what do you guys think is probably the issue here?
So, it's Newtonian mechanics, and the reason for this is because Newtonian mechanics does not work on this very, very small size scale. As we said, Newtonian mechanics does work in most cases, it does work when we're discussing things that we can see, it does work even on things that are too small to measure. But once we got to the atomic size scale, what happens is we need to be taking into account the fact that matter has these wave-like properties, and we'll learn more about that later, but essentially classical mechanics does not take that into account at all. So, we need a new kind of mechanics, which is quantum mechanics, which will accurately explain the behavior of molecules on this small scale.
So, as I mentioned, the real key to quantum mechanics is that it's treating matter not just like it's a particle, which is what we were just doing, but also like it's a wave, and it treats light that way, too. The second important point to quantum mechanics is that it actually considers the fact that light consists of these discrete packets or particle-like pieces of energy, which are called photons. And if you think about what's actually happening here, this second point that light consists of photons is actually the same thing as saying that light shows particle-like properties, but that's such an important point that I put it separately, and we'll cover that separately as we go along.
So, we now have this new way of thinking about how a nucleus and an electron can hang together, and this is quantum mechanics, and we can use this to come up with a new way to describe our atom and the behavior of atoms. But the problem is before we do this, it makes sense to take a little bit of a step back and actually make sure we're all on the same page and understanding why quantum mechanics is so important and how it works, and specifically understanding what we mean when we say that light is both a particle and a wave, and that matter is both a particle and a wave. So, we'll move on to this discussion of light as a wave, and we really won't pick up into going back to applying quantum mechanics to the atom until Friday, but in the meantime, we'll really get to understand the wave particle duality of light and of matter.
So, we'll start with thinking about some properties of waves that are going to be applicable to all waves that we're talking about, including light waves. The easiest kind of waves for us to picture are ocean waves or water waves, because we can, in fact, see them, but they have similar properties to all waves. And those properties include that you have this periodic variation of some property. So, when we're talking about water waves, the property we're discussing is just the water level.
So, for example, we have this average level, and then it can go high where we have the peak, or it can go very low. We can also discuss sound waves, so again it's just the periodic variation of some property -- in this case we're talking about density, so we have high density areas and low density areas.
So, regardless of the type of wave that we're talking about, there's some common definitions that we want to make sure that we're all able to use, and the first is amplitude. And when we're talking about the amplitude of the wave, we're talking about the deviation from that average level. So, if we define the average level as zero, you can have either a positive amplitude or a negative amplitude. So, sometimes people get confused when they're solving problems and call the amplitude this distance all the way from the max to the min, but it's only half of that because we're only going back to the average level.
So, what we really want to talk about here is light waves, and light waves have the same properties as these other kind of waves in that they're the periodic variation of some property. So, when we're discussing light waves, what we're talking about is actually light or electromagnetic radiation, is what we'll be calling it throughout the course. And that's the periodic variation of an electric field.
So, instead of having the periodic variation of water, or the periodic variation of air density, here we're talking about an electric field. We know what an electric field is, it's just a space through which a Coulomb force operates. And the important thing to think about when you're talking about the fact that it's a periodic variation, is if you put a charged particle somewhere into an electric field, it will, of course, go in a certain direction toward the charge it's attracted to. But you need to think about the difference, if you have a particle here on your wave, it will go in one direction. But remember, waves don't just have magnitude, they also do have direction. So, if instead you put your particle somewhere down here on the electric field, or on the wave, the electric field will now be in the other direction, so your particle will be pushed the other way.
And from physics you know that, of course, if we have a propagating electric field, we also have a perpendicular magnetic field that's going back and forth. But in terms of worrying about using the concepts of a wave to solve chemistry problems in this course, we can actually put aside the fact, and only focus on the electric field part of things, because that's what's going to be interacting with our charged particles, such as our electrons.
So, other properties of waves that you probably are all familiar with but I just want to review is the idea of a wavelength. If we're talking about the wavelength of a wave, we're just talking about the distance that there is between successive maxima, or of course, we can also be talking about the distance between successive minima. Basically, we can take any point on the wave, and it's the distance to that same point later on in the wave. So, that's what we call one wavelength. We also commonly discuss the frequency of a wave, and the frequency is just the number of cycles that that wave goes through per unit time. So, by a cycle we'd basically mean how many times we cycle through a complete wavelength. So, if something cycles through five wavelengths in a single second, we would just say that the frequency of that wave is five per second.
We can also mathematically describe what's going on here other than just graphing it. So, if we want to look at the mathematical equation of a wave, we want to describe -- again as I mention, what we're describing is the electric field, we're not worrying about the magnetic field here, as a function of x and t that's equal to a cosine [ 2 pi x over wavelength, minus 2 pi nu t ]. And note this is the Greek letter nu. This is not a v. Where we have E, which is equal to the electric field, what is x?
STUDENT: Position.
PROFESSOR: Yup, the position of the wave. And what about t? Yeah, so we're talking about both position and time. So what we can do if we're talking about a wave is think of it both in terms of position time, but if we're trying to visualize this -- for example if we're actually to graph this out, the easiest thing to do is keep one of these two variables constant, either the x or the t, and then just consider the other variable.
So, for example, if we're to hold the time constant, this makes it a lot simpler of an equation, because what we can end up doing is actually crossing out this whole term here. So what we're left with is just that the electric field as a function of distance is a times cosine of the argument there, which is now just 2 pi x over wavelength. So, what we want to be able to do, either when we're looking at the graph or looking at the equation up there, is to think about different properties of the wave. For example, to think about at what point do we have the wave where it's at its maximum amplitude? So, if we think about that, we need to have a point where we're making this argument of the cosine such that the cosine is going to all be equal to one, so all we're left with is that a term. So, we can do that basically any time that we have an integer variable that is either zero or an integer variable of the wavelength. So, for example, negative wavelength or positive wavelength are two times the wavelength, because that lets us cross out the term with the wavelength here, and we're left with some integer multiple of just pi.
So, that's sort of the mathematically how we get to a, but we can also just look at the graph here, because every time we go one wavelength, we can see that we're back in a maximum.
So, I mentioned we should be able to figure out where the maximum amplitude is. You should also just looking at an equation, immediately be able to figure out what that maximum amplitude is in terms of the height of it just by looking at that a-term, here we should also be able to know the intensity of any light wave, because intensity is just the amplitude squared. So, we should immediately be able to know how bright or how intense a light is just looking at the wave equation, or just by looking at a graph.
We can also do a similar thing, and I'll keep my distance from the board, but we can instead be holding x constant, for example, putting x to be equal to zero, and then all we're doing is considering the electric field as a function of t. So, in this case we're crossing out the first term there, and we're left with amplitude times the cosine of 2 pi nu times t. And, of course, we can do the same thing again, we can think about when the amplitude is going to be at its maximum, and it's going to be any time cosine of this term now is equal to one. So that will be at, for example, negative 1 over nu, or 0, or 1 over nu. And again, we can just look at our graph to figure that out, that's exactly where we're at a maximum.
So, 1 over nu is another term we use and we call it the period of a wave, and the period is just the inverse of the frequency. And if we think about frequency, that's number of cycles per unit time. So, for example, number of cycles per second, whereas the period is how much time it takes for one cycle to occur.
And when we talk about units of frequency, in almost every case, you'll be talking about number of cycles per second. So, you can just write inverse second, the cycle part is assumed. But you'll also frequently see it called Hertz, so, Hz here. So, if you're talking about five cycles per second, you can write five per second, or you can write five Hertz. The one thing you want to keep in mind though is that Hertz does not actually mean inverse seconds, it means cycles per second. So, if you're talking about a car going so many meters per second, you can't say it's going meter Hertz, you have to say meters per second. So, this really just means for frequency, it's a frequency label.
Alright. So, since we have these terms defined, we know the frequency and the wavelength, it turns out we can also think about the speed of the wave, and specifically of a light wave, and speed and is just equal to the distance that's traveled divided by the time the elapsed. And because we've defined these terms, we have ways to describe these things. So, we can describe the distance that's traveled, it's just a wavelength here. And we can think about how long it takes for a wave, because waves are, we know not just changing in position, but the whole wave is moving forward with time, we can think about how long it takes for wave to go one wavelength. So, one distance that's equal to lambda. So, how much time would that take, does anyone know?
So, would it take, for example, the same amount of time as the frequency? The period, that's right. So, it's going to take one period to move that long. And another way we can say period is just 1 over nu or 1 over the frequency So, now we know both the distance traveled and the time the elapsed. So, we can just plug it in. Speed is equal to the distance traveled, which is lambda over the time elapsed, which is 1 over nu. so, we can re-write that as speed is equal to lambda times nu, and it turns out typically this is reported in meters per second or nanometers per second.
So, now we have an equation where we know the relationship between speed and wavelength and frequency, and it turns out that we could take any wave, and as long as we know the frequency and the wavelength, we'll be able to figure out the speed. But, of course, there's something very special about electromagnetic waves, electromagnetic radiation and the speed. And it's not really surprising for me to tell you that electromagnetic radiation has a constant speed, and that speed is what we call the speed of light, and typically we abbreviate that as c, and that's from the Latin term celeritas, which means speed in Latin. That's one of four or five Latin words I remember from four years of high school Latin, but it comes in handy to remember speed of light. And some of you may have memorized what the speed of light is in high school -- it's about 3 times 10 to the 8 meters per second. This is another example of a constant that you will accidentally memorize in this course as you use it over and over again. But again, that we will supply for you on the exam just in case you forget it at that moment.
And this is a very fast speed, of course, it's about 700 million miles per hour. So, one way to put that in perspective is to think about how long it takes for a light beam to get from earth to the moon. Does anyone have any guesses? Eight seconds, that sounds good. Anyone else? These are all really good guesses, so it actually takes 1.2 seconds for light to travel from the earth to the moon. So, we're talking pretty fast, so that's nice to appreciate in itself. But other than that point, we can also think about the fact that frequency and wavelength are related in a way that now since we know the speed of light, if we know one we can tell the other. So, you can go ahead and switch us to our clicker question here.
So, we should be able to look at different types of waves and be able to figure out something about both their frequency and their wavelength, and know the relationship between the two. So, it's up on this screen here now, so we'll work on the other one. If you can identify which of these statements is correct based on what you know about the relationship between frequency and wavelength and also just looking at the waves.
Alright. So, let's give ten more seconds on that. So, ten seconds on that. Alright. So, good job. So, most people could recognize that light wave a has the shorter wavelength. We can see that just by looking at the graph itself -- we can see, certainly, this is shorter from maxima to maxima. This we can't even see the next maxima, so it's much longer. And then, we also know that means that it has the higher frequency, because our relationship between wavelength and frequency are inversely related. And also, we know the speed of light. So, if we think about if it's a shorter wavelength, we'll be able to get a lot more wavelengths in, in a given time, than we would for a longer wavelength.
So, we can switch back to the notes and think about what this means, and what this means when we're talking about all the different kinds of light waves we have, and I've shown a bunch here, is that if we have the wavelength, we also know the frequency of these wavelengths. So, for example, radio waves, which have very long wavelengths have very low frequencies. Whereas where we go to waves that have very short wavelengths, such a x-rays or cosmic rays, they, in turn, have very high frequencies.
So, it's important to get a little bit of a sense of what all these different kinds of lights do. You're absolutely not responsible to memorize what the wavelengths of the different types of lights are, but you do want to be able to know the general order of them. So, if someone tells you they're using UV light versus x-ray light, you know that the x-ray light is, in fact, at a higher frequency. So that's the important take-away message from this slide. If we think about these different types of lights, microwave light, if it's absorbed by a molecule, is a sufficient amount of frequency and energy to get those molecules to rotate. That, of course, generates heat, so that's how your microwaves work.
If we talk about infrared light, which is at a higher frequency here and a shorter wavelength, infrared light when it's absorbed by molecules actually is enough to cause molecules now to vibrate. If we move up to the more high-frequency and divisible light and all the way into UV light, if you shine UV light at certain molecules, it's going to have enough energy to actually pop those electrons in that molecule up to a higher energy level, which will make more sense once we talk about energy levels in atoms, but that's what UV light can do. And actually, that's responsible for fluorescence and phosphorescence that you see where typically UV light comes in. So, if you use a black lamp or something and you excite something up to a higher energy level and then it relaxes back down to its lower energy state, it's going to emit a new wavelength of light, which is going to be visible to you.
X-rays are at even a higher frequency, and those are sufficient to actually be absorbed by a molecule and pop an electron all the way out of that molecule. You can see how that would be damaging to the integrity of that molecule, that's why x-rays are so damaging -- you don't want to have electrons disappearing for no good reason from your molecules that can cause the kind of mutations we don't want to be seeing in ourselves. And then also as we go higher, we have gamma rays and cosmic rays.
Within the visible range of what we can see, you also want to know this relative order that's pretty easy -- most of us have memorized that in kindergarten, so that should be fine. Just remembering that violet is the end that actually has the shortest wavelength, which means that it also has, of course, the highest frequency.
So, just an interesting fact about this set of light, which we're most familiar with, if we think about our vision, it turns out that our vision's actually logarithmic and it's centered around this green frequency. So, if instead of a red laser pointer here, I had a green one, you'd actually, to our eyes, it would seem like the green one was brighter, even if the intensity was the same, and that's just because our eyes are centered and logarithmic around this green frequency set.
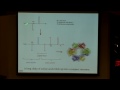
So, using the relationship between frequency and wavelength, we can actually understand a lot about what's going on, and pretty soon we'll also draw the relationship very soon to energy, so it will be even more informative then. But I just want to point out one of the many, many groups at MIT that works with different fluorescing types of molecules, and this is Professor Bawendi's laboratory at MIT, and he works with quantum dots. And quantum dots are these just very tiny, tiny crystals of semiconductor material. They're on the order of one to ten nanometers, and these can be shined on with UV light -- they have a lot of different interesting properties, but one I'll mention is that if you excite them with UV light, they will have some of the electrons move to a higher energy state, and when they drop back down, they actually emit light with a wavelength that corresponds with the size of the actual quantum dot.
So, from what we know so far, we should be able to look at any of these quantum dots, which are depicted as a cartoon here, but here we have an actual picture of the quantum dots suspended in some sort of solution and shone on with UV light, and you can see that you can achieve this whole beautiful range of colors just by modulating the size of the different dots. And we should be able to know if we're looking at a red dot -- is a red dot, it's going to have a longer wavelength, so is this a higher or lower frequency? Yeah, and similarly, if someone tells us that their dot is blue-shifted, that should automatically in our heads tell us, oh it shifted to a higher frequency.
And these dots are really interesting in that you can, I'm sure by looking at this picture, already imagine just a whole slew of different biological or sensing applications that you could think of. For example, if you were trying to study different protein interactions, you could think about labeling them with different colored dots, or there's also a bunch of different fluorescent techniques that you could apply using these dots, or you could think of in-vivo sensing, how useful these could be if you could think of a way to get them into your body without being too toxic, for example.
These are all things that the Bawendi group is working on. What they are real experts in is synthesizing many different kinds of these dots, and they have a synthetic scheme that's used by research groups around the world. The Bawendi group also collaborates with people, both at different schools and at MIT. One example, on some of their biochemistry applications is with another Professor at MIT, Alice Ting and her lab.
So really what I want to point out here is as we get more into describing quantum mechanics, these quantum dots are one really good example where a lot of the properties of quantum mechanics apply directly. So, if you're interested, I put the Bawendi lab research website onto your notes. And also, Professor Bawendi recently did an interview with "The Tech." Did anyone see that interview in the paper? So, three or four -- a few of you read the paper last week. So, you can either pick up an old issue or I put the link on the website, too. And that's not just about his research, it's also about some of his memories as a student and advice to all of you. So, it's interesting to read and get to know some of these Professors at MIT a little bit better.
So, one property that was important we talked about with waves is the relationship between frequency and wavelength. Another very important property of waves that's true of all waves, is that you can have superposition or interference between two waves. So, if we're looking at waves and they're in-phase, and when I talk about in-phase, what I mean is that they're lined up, so that the maxima are in the same position and the minima are in the same position, what we can have a something called constructive interference. And all we mean by constructive interference is that literally those two waves add together, such as the maxima are now twice as high, and the minima are now twice as low.
So, you can also imagine a situation where instead of being perfectly lined up, now we have the minima being lined up with the maxima here. So, if we switch over to a clicker question maybe on this screen -- okay, can it be done up there to switch? So, we're still settling in with the renovations here in this room. So, why don't you all go ahead and tell me what happens if you combine these two waves, which are now out of phase?
So, let's -- okay, so, why don't you all think about would happen -- we'll start with the thought exercise. You can switch back to my lecture notes then if this isn't going. Alright. So, hopefully what everyone came up with is the straight line, is that what you answered?
STUDENT: Yeah.
PROFESSOR: OK, very good. And I didn't make you try to draw the added, the superimposed positive construction in your notes, but I think everyone can handle drawing a straight line. So, you can go ahead and draw what happens when we have destructive interference. And destructive interference, of course, is the extreme, but you can picture also a case where you have waves that are not quite lined up, but they're also not completely out of phase. So in that case, you're either going to have the wave get a little bigger, but not twice as big or a little bit smaller.
So, I think the easiest way to think about interference is not actually with light, but sometimes it's easiest to think about with sound, especially when you're dealing with times where you have destructive interference. Has anyone here ever been in a concert hall where they feel like they're kind of in a dead spot, or you don't quite hear as well, and if you move down just two seats all of a sudden it's just blasting at you -- hopefully not in this room. But have people experienced that before? Yeah, I definitely experienced it, too. And really, all you're experiencing there is destructive interference in a very bad way. Halls, they try to design halls such that that doesn't happen, and I show an example of a concert hall here -- this is Symphony Hall in Boston, and I can pretty much guarantee you if you do go to this Symphony Hall, you will not experience a bad seat or a dead seat. This is described as actually one of the top two or three acoustic concert halls in the whole world. So, it's very well designed such that they've minimized any of these destructive interference dead sounds. So, it's nice, on a student budget you can go and get the worst seat in the house and you can hear just as well as they can hear up front, even if you can't actually see what's going on.
So, another example of destructive interference is just with the Bose headphones. I've never actually tried these on, but you see people with them, and what happens here is it's supposed to be those noise cancellation headphones. All they do is they take in the ambient noise that's around it, and there's actually battery in the headphones, that then produces waves that are going to destructively interfere with that ambient noise. And that's how it actually gets to be so quiet when you have on, supposedly, these quite expensive headphones.
So, that's light as a wave, and the reason -- well, that was sound as a wave, but light as a wave is the same idea. And it was really established by the early 1900s that, in fact, light behaved as a wave. And the reason that it was so certain that light was a wave was because we could observe these things -- we could see, for example, that light defracted, and we could see that light constructively or destructively could interfere with other light waves, and this was all confirmed and visualized. But also, around the time that Thomson was discovering the electron, there were some other observations that were going on, and the most disturbing to kind of the understanding of the universe was the fact that there were some observations about light that didn't make sense with this idea that light is a particle. And the photoelectric effect is maybe the most clear example of this.
So, the photoelectric effect is the effect that if you have some metal, and you can pick essentially any metal you want, and you shine light of a certain frequency onto that metal, you can actually pop off an electron, and you can go ahead and measure what the kinetic energy of that electron that comes off is, because we can measure the velocity and we know that kinetic energy equals 1/2 m b squared, and thanks to Thomson we also know the mass of an electron.
So, this is an interesting observation, and in itself not too disturbing, yet but the important thing to point out is that there's this threshold frequency that is of the metal, and each metal has a different threshold frequency, such as if you shine light on the metal where the frequency of the light is less than the threshold frequency, nothing will happen -- no electron will pop off of that metal. However, if you shine a light with a frequency that's greater than the threshold frequency, you will be able to pop off an electron.
So, people were making this observation, but this wasn't making any sense at all because there was nothing in classical physics that described any sort of relationship between the frequency of light and the energy, much less the energy of an electron that would get popped off of a metal that would basically come off only when we're hitting this threshold frequency. So, what they could do was actually graph what was happening here, so we can also graph what was happening, and what they found was that if we were at any point below the threshold frequency and we were counting the numbers of electrons that were popping off of our metal, we weren't seeing anything at all. But if you go up the threshold frequency, suddenly you see that there's some number of electrons that comes off, and amazingly, the number of electrons actually had no relationship at all to the frequency of the light.
And this didn't make a lot of sense to people at the time because they thought that the frequency should be related to the number of electrons that are coming off, because you have more frequency coming in, you'd expect more electrons that are coming off -- this wasn't what people were seeing. So, what they decided to do is just study absolutely everything they could about the photoelectric effect and hope, at some point, someone would piece something together that could explain what's going on or shed some light on this effect.
So, one thing they did, because it was so easy to measure kinetic energy of electrons, is plot the frequency of the light against the kinetic energy of the electron that's coming off here. And in your notes and on these slides here, just for your reference, I'm just pointing out what's going to be predicted from classical physics. You're not responsible for that and we won't really discuss it, but it just gives you the contrast of the surprise that comes up when people make these observations. And the first observation was that the frequency of the light had a linear relationship to the kinetic energy of the electrons that are ejected here. This made no sense at all to people, and again they saw this effect where if you were below that threshold frequency, you saw nothing at all.
So, that was frequency with kinetic energy. The next thing that they wanted to look at was the actual intensity of the light and see what the relationship of intensity to kinetic energy is. So, what we would expect is that there is a relationship between intensity in kinetic energy, because it was understood that however intense the light was, if you had a more intense light, it was a higher energy light beam. So that should mean that the energy that's transferred to the electron should be greater, but that's not what you saw at all, and what you saw is that if you kept the frequency constant, there was absolutely no change in the kinetic energy of the electrons, no matter how high up you had the intensity of the light go. You could keep increasing the intensity and nothing was going to happen.
So, we could also plot the number of electrons that are ejected as a relationship to the intensity, so that was yet another experiment they could do. And this is what they had expected that there would be no relationship, but instead here they saw that there was a linear relationship not to the intensity and the kinetic energy of the electrons, but to the intensity and the number of electrons.
So, none of these observations made sense to any scientists at the time, and really all of these observations were made and somewhat put aside for several years before someone that could kind of process everything that was going on at once came along, and that person was Einstein, conveniently enough -- if anyone could put it together, we would hope that he could, and he did. And what he did in a way that made sense when all of us look at it, is he plotted all of these different metals on the same graph and made some observations. So, for example, here we're showing rubidium and potassium and sodium plotted where we're plotting the frequency -- that's the frequency of that light that's coming into the metal versus the kinetic energy of the electron that's ejected from the surface of the metal.
And what he found here, which is what you can see and we can all see pretty clearly, is the slope of all of these lines is the same regardless of what the type of metal is. So, he fit all these to the equation of the line, and what he noticed was the slope was specifically this number, 6.626 times 10 to the negative 34, joules times seconds. And he also found that the y intercept for each one of these metals was equal to basically this number here, which was the slope times the minimum frequency required of each specific metal, so that's of the threshold frequency.
And he actually knew that this number had popped up before, and a lot of you are familiar with this number also, and this is Planck's constant. Planck had observed this number as a fitting constant years earlier when he looked at some phenomena, and you can read about in your book, such as black body radiation. And what he found was he needed this constant to fit his data to what was observed. And this is the same thing that Einstein was observing, that he needed this fitting constant, that this constant was just falling right out of, for example, this slope and also the y intercept. So he decided to go ahead and define exactly what it is, this line, in terms of these new constants, this constant he's calling h, which is Planck's constant. So, on the y axis we have kinetic energy, so we can plug that in. If we talk about what the x axis is, that's just the frequency of the light that's coming in. We know what m is, m is equal to h. And then we can plug in what b is, the y intercept, because that's just the negative of h times that threshold frequency.
So we have this new equation here when we're considering this photoelectric effect, which is that the kinetic energy is equal to h nu minus h nu threshold of the metal. And what Einstein concluded and observed is that well, kinetic energy, of course, that's an energy term, and h times nu, well that has to be energy also, because energy has to be equal to energy -- there's no other way about it. And this worked out with units as well because we're talking about joules for kinetic energy, and when we're talking about h times nu, we're talking about joules times second times inverse seconds. So, the very important conclusion that Einstein made here is that energy is equal to h times nu, or that h times nu is an actual energy term.
And this kind of went along with two observations. The first is that energy of a photon is proportional to its frequency. So this was never recognized before that if we know the frequency of a photon or a wave of light, we can know the energy of that light. So, since we know that there's relationship also between frequency and wavelength, we can do the same thing -- if we know the wavelength, we can know the energy of the light.
And I use the term photon here, and that's because he also concluded that light must be made up of these energy packets, and each packet has that h, that Planck's constant's worth of energy in it, so that's why you have to multiply Planck's constant times the frequency. Any frequency can't have an energy, you have to -- you don't have a continuum of frequencies that are of a certain energy, it's actually punctuated into these packets that are called photons. And, as you know, Einstein made many, many, many very important contributions to science and relativity, but he called this his one single most important contribution to science, the relationship between energy and frequency and the idea of photons.
So this means we now have a new way of thinking about the photoelectric effect, and that is the idea that h times nu is actually an energy. So, it's the energy of an incident photon if we're talking about nu where we're talking about the energy of the photon going in, so we can abbreviate that as e sub i, energy of the incident photon. We can talk about also h times nu nought, which is that threshold frequency. So this is a term we're going to see a lot, especially in your problem sets, it's called the work function, and the work function is the same thing as the threshold frequency of a metal, except, of course, that it's multiplied by Planck's constant. So, it's the minimum energy that a certain metal requires in order to pop a photon out of it -- in order to eject an electron from the surface of that metal.
So this is our new kind of schematic way that we can think about looking at the photoelectric effect, so if this is the total amount of energy that we put into the system, where here we have the energy of a free electron. We have this much energy going in, the metal itself requires this much energy, the work function, in order to eject an electron. So that much energy is going to be used up just ejecting it. And what we have left over is this amount of energy here, which is going to be the kinetic energy of the ejected electron. So, therefore, we can rewrite our equation in two ways. One is just talking about it in terms only of energy where our kinetic energy here is going to be equal to the total energy going in -- the energy initial minus this energy of the work function here. We can also talk about it in terms of if we want to solve, if we, for example, we want to find out what that initial energy was, we can just rearrange our equation, or we can look at this here where the initial energy is equal to kinetic energy plus the work function.
So before we go we'll try to see if we can do a clicker question for you on this, and we can, very good. So, everyone take those clickers back out and tell me, if a beam of light with a certain energy, and we're going to say four electron volts strikes a gold surface, and here we're saying that the gold surface has a work function of 5.1 electron volts, what is the maximum kinetic energy of the electron that is ejected?
So why don't you go ahead and take ten seconds on that. And if you don't know, that's okay, just type in an answer and give it your best shot. And let's see what we come up with here. Alright. So, it looks like some of you were tricked, but many of you were not, so no electrons will be ejected. The reason for that is because this is the minimum amount of energy -- hold off a sec on the packing up, so in case someone doesn't understand -- this is the minimum amount of energy that's required from the energy going in in order to eject an electron. So if the incident energy is less than the energy that's required, absolutely nothing will happen. That's the same thing we were talking about with threshold frequency.
All right, now you can pack up and we'll see you on Wednesday.
Free Downloads
Video
- iTunes U (MP4 - 108MB)
- Internet Archive (MP4 - 107MB)
Free Streaming
Subtitle
- English - US (SRT)