Welcome to the mud art gallery. Keep them coming. I will post only the highest quality stuff and all decisions are final.

General comments
Good lecture today. The PRS responses were solid. People seem to be getting the hang of identifying work and heat for various legs of a cycle, and people seem to be getting more comfortable with working with the various processes on a thermodynamic diagram.
Why is it so cold in 35-225? I don't mean from a thermodynamic point of view... I mean from a "too-much A.C." point of view. (1 student) It seems okay to me, but if several people are uncomfortable, I can try to have the internal energy increased.
Responses to 'Muddiest Part of the Lecture Cards'
(61 respondents)
1) "Design variables" ?! ( 1 student) Design variables are parameters that for one of several reasons have become commonly used in the design or assessment of engine performance. Frequently they are non-dimensional. Usually they are particularly relevant as technology limiters or benchmarks (for example the pressure ratio and maximum cycle temperature for a gas turbine engine). Sometimes they are just a convenient reference point (the atmospheric temperature for instance).
2) How do you calculate work for constant pressure heat addition? (1 student) w=p(Dv) for constant pressure heat addition.
3)What are some examples with the change in design parameters? (1 student) The plots of efficiency and work for the Otto and Brayton cycles are examples of the variation in performance with changing design parameters. Charts like these are very useful for understanding the overall performance of an engine, what can be done to improve it, etc.
4) Where does the spark come from? It comes from a spark plug (a high voltage is applied across a small air gap causing it to arc and produce a spark). Why are diesel engines louder than others? I don't know. Where exactly can we access the MIT engine (what website)? (1 student) Didn't you read the notes? Check out the end of Chapter 5 or click here.
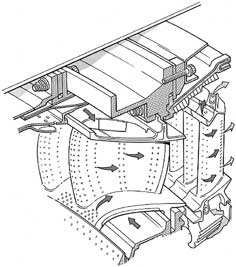
5) What was that T3 thing? I got lost in that part of the discussion. (2 students) T3 is an important design parameter for gas turbine engines. It is the turbine inlet temperature (the highest temperature in the cycle). As you can see from the performance plots for the Brayton cycle increasing the temperature ratio (T3/T1 where T1 is the atmospheric temperature) significantly increases the amount of work that can be derived from the engine per unit of mass flow. This is often desirable because at fixed thrust you can have a smaller engine (less drag, lighter, cheaper, etc.). Therefore, the gas turbine engine industry works very hard to find ways to increase the maximum allowable temperature in the cycle. So much so in fact that current engines operate with temperatures several hundred K above the melting temperature of the metals through which the gases flow. Just how hot is this "cool" air that keeps the turbine from melting? (1 student) The metal surfaces (blades, etc.) are protected by a layer of "cool" air (it is about 600-800K) that flows out of thousands of small holes on the surfaces of the parts. Here is a picture I took from a from Rolls-Royce book to give you an idea of what the inside of a turbine looks like.
Copyright Rolls-Royce plc. Reproduced with the kind permission of Rolls-Royce plc.
6) Cycle efficiency and thermal efficiency (1 student). My apologies. These are one and the same. I just use to two words interchangeably.
7) Where in the engine does each point of the graph occur? (1 student). First look at this.The only parts that (I hope) are still unclear is where the boundaries are between the inlet/compressor and the turbine/nozzle. These boundaries are a function of the details of the design of the device and the flight Mach number. During the spring semester propulsion lecture, you will learn about all of these details. For now just take the inlet and compressor together as one quasi-static, adiabatic compression and the turbine and nozzle together as one quasi-static, adiabatic expansion.
8) If no work is done by the engine to cool the exhaust gas and bring it back to the inlet (because it doesn't happen) why is this (-) work included in the p-v diagram? and related questions (3 students). Good question. From the perspective of the engine, it continuously receives cold gas in the inlet and continuously expels hot gas in the exhaust. So this part really does happen. Now what about the work? Well it really happens as well. You will learn next week that the work done during constant pressure heating or cooling is what is called "flow work" rather than "shaft work". It is the work the flow does against the atmosphere in compressing or expanding. So where the work appears in nature is that the gas that comes out of the engine occupies a larger volume than the gas that comes into the engine. So there is some net displacement of volume at constant pressure (i.e. work).
9) Why does a diesel engine have to warm up before you turn it on? (1 student) How does a two stroke engine work and why are they less efficient/pollute more? (1 student) I am not an expert in these areas. Check out Heywood's book on internal combustion engines. It is a well known reference on these topics. Or a mechanic would probably be able to tell you.
10) Will we usually be given all temperatures or will we be given any combination of values? (1 student) You will usually be given one complete state at some location in the cycle and then just enough information to get the rest (usually through specifying the various paths and a few other properties along the way). So you need to become comfortable with applying the first law and the ideal gas law to the various thermodynamic processes.
11) In deriving the w-cycle you said that q2-3=cp(T3-T2) which is Dh. Is it true that q=Dh? If so when and why? (1 student) dh=dq+vdp for a quasi-static process, cpdT=dq+vdp for a quasi-static process involving an ideal gas, and cpdT=dq for a constant pressure quasi-static process involving an ideal gas.
12) In your notes you have T-s diagrams. What is s? (Is it heat?) (1 student) No it is not heat. It is entropy. Those diagrams are legacies from when I used to cover more of the Second Law of Thermodynamics in Unified than I do now. We will get to entropy--what it is, why we need it, etc., but it won't be until the last lecture or two of thermodynamics.
13) I don't understand anything in the drawing of the typical military engine that you put up, is that a problem? (2 students) No it is not a problem, because we are not going to discuss the details of the inner workings of gas turbine engines until we get to the propulsion lectures in spring term. However, you should be familiar with the basics (a gas turbine is composed of a compressor, a combustor, and a turbine, etc.).
14) I don't understand how pressure is kept constant when heat is added in the Brayton cycle. (1 student) The gas is allowed to expand.
15) How can Qin=W? This would make the efficiency 100% which is impossible. Right. I think I wrote Q=W (or Qcycle=Wcycle). Qin does not equal Wcycle. Also, cpdT is used for constant pressure right? On the board you wrote cvdT=w for a constant pressure leg of a gas turbine cycle. (1 student) No. I gave that explanation too fast -w=cvDT for the q-s adiabatic leg. For the constant pressure leg work is pDv. But you seem to be a little confused about cp and cv. cpdT=dh and cvdT=du for an ideal gas for any process. T and u are both functions of the state of the system and independent of path. cp also happens to be the specific heat at constant pressure (number of Joules required to increase the temperature of 1kg by 1K for a constant pressure process), and cv is the specific heat at constant volume (numner of Joules required to increase the temperature of 1kg 1K for a constant volume process).
16) Is gas entering a gas turbine engine accelerated much by the compressors? How does the change in speed of air from inlet to burner compare with the speed change from burner to nozzle. (1 student). The gas entering a turbine engine is either accelerated or decelerated by the inlet depending on flight speed to a roughly constant axial velocity through the compressor (Mach=0.5). In the combustor the flow is slowed down significantly (in fact regions of reverse flow are required to hold the flame without it blowing out), the flow is then accelerated to between M=1 and M=0.5 for passage through the turbine. In the nozzle the flow is accelerated to M=1 or higher. We will learn more about this during the spring semester propulsion lectures.
17) Can you explain the warm-up question in detail? I am still confused. Is the pressure you provided the system pressure? (1 student) Leg 1 is adiabatic compression so Q=0 and W<0. Leg 2 is constant pressure expansion so Q>0, W>0. Leg 3 is isothermal expansion so Q>0, W>0. Leg 4 is constant volume cooling so Q<0, W=0. So none of the descriptions of the legs is correct so the correct answer is (5) none of the above.
18) How does an afterburner work? What does it do? (1 student) An afterburner converts chemical energy (fuel+oxidizer) to internal energy in the gas. This internal energy then is converted to kinetic energy in the nozzle giving a very high jet velocity and thus lots of thrust. It is a way to get a quick boost of thrust (but the efficiency is very poor). During spring semester, you will probably get a chance to calculate the details of a cycle with an afterburner in it.
19) What exactly do engines do? Provide power and energy to get all the other stuff in the aircraft to work? And how do they connect? (1 student) The primary function of the engines is to propel the vehicle. They also supply power to run some of the other equipment on the aircraft. They are connected via thrust mounts that carry the load to the aircraft structure.
20) In a spark ignition engine, is a spark generated every time the piston moves up and down? (1 student) Not for a four stroke engine. The spark plug only fires when there is a compressed charge of fuel and air. It does not fire on the exhaust stroke.
21) What is the difference between power efficiency for the cycles and thermal efficiency. (1 student) I do not know what power efficiency is. Stick to thermal efficiency.
22) Higher T3/T1 higher efficiency? (1 student). No. Ideal efficiency is only a function of the temperature ratio across the compressor (T2/T1). Raising the turbine inlet temperature (T3) increases the work per unit of mass flow.
23) Not quite sure how the -pdv and +vdp parts of the du and dh equations deal with an ideal gas (1 student). The appearance of these terms in the First Law equations signals that the equation is valid for a quasi-static process. The appearance of du=cvdT and dh=cpdT signals that they are valid for an ideal gas.
24) An equation list would be helpful (1 student) It is still on my to do list.
25) No mud (31 students). Wow. I think that is an all-time high for me this semester.